Evaluation of the BioFire FilmArray Pneumonia Panel for Detection of Viral and Bacterial Pathogens in Lower Respiratory Tract Specimens in the Setting of a Tertiary Care Academic Medical Center
- PMID: 32321782
- PMCID: PMC7315030
- DOI: 10.1128/JCM.00343-20
Evaluation of the BioFire FilmArray Pneumonia Panel for Detection of Viral and Bacterial Pathogens in Lower Respiratory Tract Specimens in the Setting of a Tertiary Care Academic Medical Center
Abstract
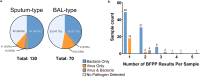
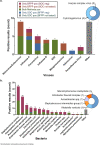
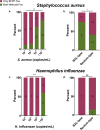
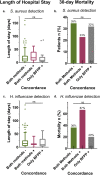
Our objective was to evaluate the diagnostic yield and accuracy of the BioFire FilmArray pneumonia panel (BFPP) for identification of pathogens in lower respiratory tract specimens (n = 200) from emergency department (ED) and intensive care unit (ICU) patients at a tertiary care academic medical center. Specimens were collected between January and November 2018, from patients ≥18 years of age, and culture was performed as part of standard-of-care testing. The BFPP identified a viral or bacterial target in 117/200 (58.5%) samples, including Staphylococcus aureus in 22% of samples and Haemophilus influenzae in 14%, and both a viral and bacterial target in 4% of samples. The most common viruses detected by BFPP were rhinovirus/enterovirus (4.5%), influenza A virus (3%), and respiratory syncytial virus (RSV) (2%). Overall, there was strong correlation between BFPP and standard methods for detection of viruses (99.2%) and bacteria (96.8%). Most bacteria (60/61 [98.4%]) detected by standard methods were also identified by BFPP, and 92 additional bacteria were identified by BFPP alone, including 22/92 (23.9%) additional S. aureus isolates and 25/92 (27.2%) H. influenzae isolates, which were more frequently discordant when detected at low concentrations (S. aureus, P < 0.001; H. influenzae, P < 0.0001) and in sputum-type specimens (S. aureus, P < 0.05). A potential limitation of the BFPP assay is the absence of fungal targets and Stenotrophomonas maltophilia, which were detected in 26 and 4 of 200 specimens, respectively. Real-time specimen analysis with BFPP has the potential to identify bacterial pathogens and resistance markers 44.2 and 56.3 h faster than culture-based methods. The BFPP is a rapid and accurate method for detection of pathogens from lower respiratory tract infections.
Keywords: BioFire; pneumonia; quantitative PCR; syndromic testing.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- WHO. 2019. World health statistics 2019: monitoring health for the SDGs, sustainable development goals. World Health Organization, Geneva, Switzerland.
-
- Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. 2019. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 200:e45–e67. doi: 10.1164/rccm.201908-1581ST. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

