Discrete Virus Factories Form in the Cytoplasm of Cells Coinfected with Two Replication-Competent Tagged Reporter Birnaviruses That Subsequently Coalesce over Time
- PMID: 32321810
- PMCID: PMC7307154
- DOI: 10.1128/JVI.02107-19
Discrete Virus Factories Form in the Cytoplasm of Cells Coinfected with Two Replication-Competent Tagged Reporter Birnaviruses That Subsequently Coalesce over Time
Abstract
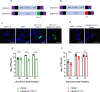
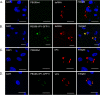
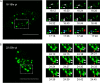
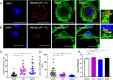
The Birnaviridae family, responsible for major economic losses to poultry and aquaculture, is composed of nonenveloped viruses with a segmented double-stranded RNA (dsRNA) genome that replicate in discrete cytoplasmic virus factories (VFs). Reassortment is common; however, the underlying mechanism remains unknown given that VFs may act as a barrier to genome mixing. In order to provide new information on VF trafficking during dsRNA virus coinfection, we rescued two recombinant infectious bursal disease viruses (IBDVs) of strain PBG98 containing either a split GFP11 or a tetracysteine (TC) tag fused to the VP1 polymerase (PBG98-VP1-GFP11 and PBG98-VP1-TC). DF-1 cells transfected with GFP1-10 prior to PBG98-VP1-GFP11 infection or stained with a biarsenical derivative of the red fluorophore resorufin (ReAsH) following PBG98-VP1-TC infection, had green or red foci in the cytoplasm, respectively, that colocalized with VP3 and dsRNA, consistent with VFs. The average number of VFs decreased from a mean of 60 to 5 per cell between 10 and 24 h postinfection (hpi) (P < 0.0001), while the average area increased from 1.24 to 45.01 μm2 (P < 0.0001), and live cell imaging revealed that the VFs were highly dynamic structures that coalesced in the cytoplasm. Small VFs moved faster than large (average 0.57 μm/s at 16 hpi compared to 0.22 μm/s at 22 hpi), and VF coalescence was dependent on an intact microtubule network and actin cytoskeleton. During coinfection with PBG98-VP1-GFP11 and PBG98-VP1-TC viruses, discrete VFs initially formed from each input virus that subsequently coalesced 10 to 16 hpi, and we speculate that Birnaviridae reassortment requires VF coalescence.IMPORTANCE Reassortment is common in viruses with segmented double-stranded RNA (dsRNA) genomes. However, these viruses typically replicate within discrete cytoplasmic virus factories (VFs) that may represent a barrier to genome mixing. We generated the first replication competent tagged reporter birnaviruses, infectious bursal disease viruses (IBDVs) containing a split GFP11 or tetracysteine (TC) tag and used the viruses to track the location and movement of IBDV VFs, in order to better understand the intracellular dynamics of VFs during a coinfection. Discrete VFs initially formed from each virus that subsequently coalesced from 10 h postinfection. We hypothesize that VF coalescence is required for the reassortment of the Birnaviridae This study provides new information that adds to our understanding of dsRNA virus VF trafficking.
Keywords: IBDV; birnavirus; coinfection; double-stranded RNA virus; reassortment; viroplasm; virus factory.
Copyright © 2020 Campbell et al.
Figures



References
-
- Cazaban C, Gardin Y, Oort RV. 2017. Gumboro disease: a persisting problem. Ceva Santé Animale, Libourne, France.
-
- Eterradossi N, Saif YM. 2013. Infectious bursal disease In Swayne DE. (ed), Diseases of poultry, 13th ed John Wiley & Sons, Inc, New York, NY.
-
- Ulrich K, Wehner S, Bekaert M, Di Paola N, Dilcher M, Muir KF, Taggart JB, Matejusova I, Weidmann M. 2018. Molecular epidemiological study on infectious pancreatic necrosis virus isolates from aquafarms in Scotland over three decades. J Gen Virol 99:1567–1581. doi: 10.1099/jgv.0.001155. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007037/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007034/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001845/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007038/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

