Single-cell O2 exchange imaging shows that cytoplasmic diffusion is a dominant barrier to efficient gas transport in red blood cells
- PMID: 32321831
- PMCID: PMC7211990
- DOI: 10.1073/pnas.1916641117
Single-cell O2 exchange imaging shows that cytoplasmic diffusion is a dominant barrier to efficient gas transport in red blood cells
Abstract
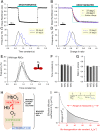
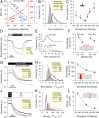
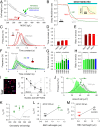
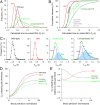
Disorders of oxygen transport are commonly attributed to inadequate carrying capacity (anemia) but may also relate to inefficient gas exchange by red blood cells (RBCs), a process that is poorly characterized yet assumed to be rapid. Without direct measurements of gas exchange at the single-cell level, the barriers to O2 transport and their relationship with hematological disorders remain ill defined. We developed a method to track the flow of O2 in individual RBCs by combining ultrarapid solution switching (to manipulate gas tension) with single-cell O2 saturation fluorescence microscopy. O2 unloading from RBCs was considerably slower than previously estimated in acellular hemoglobin solutions, indicating the presence of diffusional barriers in intact cells. Rate-limiting diffusion across cytoplasm was demonstrated by osmotically induced changes to hemoglobin concentration (i.e., diffusive tortuosity) and cell size (i.e., diffusion pathlength) and by comparing wild-type cells with hemoglobin H (HbH) thalassemia (shorter pathlength and reduced tortuosity) and hereditary spherocytosis (HS; expanded pathlength). Analysis of the distribution of O2 unloading rates in HS RBCs identified a subpopulation of spherocytes with greatly impaired gas exchange. Tortuosity imposed by hemoglobin was verified by demonstrating restricted diffusivity of CO2, an acidic gas, from the dissipative spread of photolytically uncaged H+ ions across cytoplasm. Our findings indicate that cytoplasmic diffusion, determined by pathlength and tortuosity, is a major barrier to efficient gas handling by RBCs. Consequently, changes in RBC shape and hemoglobin concentration, which are common manifestations of hematological disorders, can have hitherto unrecognized and clinically significant implications on gas exchange.
Keywords: diffusion; erythrocyte; fluorescence; gas channels; oxygen.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Assessing the kinetics of oxygen-unloading from red cells using FlowScore, a flow-cytometric proxy of the functional quality of blood.EBioMedicine. 2025 Jan;111:105498. doi: 10.1016/j.ebiom.2024.105498. Epub 2024 Dec 14. EBioMedicine. 2025. PMID: 39674089 Free PMC article.
-
Red blood cell thickness is evolutionarily constrained by slow, hemoglobin-restricted diffusion in cytoplasm.Sci Rep. 2016 Oct 25;6:36018. doi: 10.1038/srep36018. Sci Rep. 2016. PMID: 27777410 Free PMC article.
-
Calculations of oxygen transport by red blood cells and hemoglobin solutions in capillaries.Artif Cells Blood Substit Immobil Biotechnol. 2002 May;30(3):157-88. doi: 10.1081/bio-120004338. Artif Cells Blood Substit Immobil Biotechnol. 2002. PMID: 12066873
-
Control and consequences of adrenergic activation of red blood cell Na+/H+ exchange on blood oxygen and carbon dioxide transport in fish.J Exp Zool. 1992 Aug 15;263(2):160-75. doi: 10.1002/jez.1402630206. J Exp Zool. 1992. PMID: 1323642 Review.
-
Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport.Acta Physiol Scand. 2004 Nov;182(3):215-27. doi: 10.1111/j.1365-201X.2004.01361.x. Acta Physiol Scand. 2004. PMID: 15491402 Review.
Cited by
-
The volume of healthy red blood cells is optimal for advective oxygen transport in arterioles.Biophys J. 2024 May 21;123(10):1289-1296. doi: 10.1016/j.bpj.2024.04.015. Epub 2024 Apr 18. Biophys J. 2024. PMID: 38641875 Free PMC article.
-
Stored blood has compromised oxygen unloading kinetics that can be normalized with rejuvenation and predicted from corpuscular side-scatter.Haematologica. 2022 Jan 1;107(1):298-302. doi: 10.3324/haematol.2021.279296. Haematologica. 2022. PMID: 34498445 Free PMC article. No abstract available.
-
Single-cell oxygen saturation imaging shows that gas exchange by red blood cells is not impaired in COVID-19 patients.Br J Haematol. 2020 Aug;190(4):e229-e232. doi: 10.1111/bjh.17025. Epub 2020 Aug 1. Br J Haematol. 2020. PMID: 32678950 Free PMC article. No abstract available.
-
Assessing the kinetics of oxygen-unloading from red cells using FlowScore, a flow-cytometric proxy of the functional quality of blood.EBioMedicine. 2025 Jan;111:105498. doi: 10.1016/j.ebiom.2024.105498. Epub 2024 Dec 14. EBioMedicine. 2025. PMID: 39674089 Free PMC article.
-
Assessment of transient changes in oxygen diffusion of single red blood cells using a microfluidic analytical platform.Commun Biol. 2021 Mar 2;4(1):271. doi: 10.1038/s42003-021-01793-z. Commun Biol. 2021. PMID: 33654170 Free PMC article.
References
-
- Wagner P. D., Diffusion and chemical reaction in pulmonary gas exchange. Physiol. Rev. 57, 257–312 (1977). - PubMed
-
- Boron W. F., Endeward V., Gros G., Musa-Aziz R., Pohl P., Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels. ChemPhysChem 12, 1017–1019 (2011). - PubMed
-
- Chakraborty S., Balakotaiah V., Bidani A., Diffusing capacity reexamined: Relative roles of diffusion and chemical reaction in red cell uptake of O2, CO, CO2, and NO. J. Appl. Physiol. 97, 2284–2302 (2004). - PubMed
-
- Geers C., Gros G., Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol. Rev. 80, 681–715 (2000). - PubMed
-
- Gros G., Moll W., The diffusion of carbon dioxide in erythrocytes and hemoglobin solutions. Pflugers Arch. 324, 249–266 (1971). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

