Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling
- PMID: 32321834
- PMCID: PMC7211957
- DOI: 10.1073/pnas.1919702117
Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling
Abstract
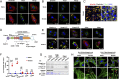
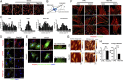
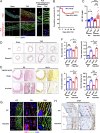
The extracellular matrix (ECM) initiates mechanical cues that activate intracellular signaling through matrix-cell interactions. In blood vessels, additional mechanical cues derived from the pulsatile blood flow and pressure play a pivotal role in homeostasis and disease development. Currently, the nature of the cues from the ECM and their interaction with the mechanical microenvironment in large blood vessels to maintain the integrity of the vessel wall are not fully understood. Here, we identified the matricellular protein thrombospondin-1 (Thbs1) as an extracellular mediator of matrix mechanotransduction that acts via integrin αvβ1 to establish focal adhesions and promotes nuclear shuttling of Yes-associated protein (YAP) in response to high strain of cyclic stretch. Thbs1-mediated YAP activation depends on the small GTPase Rap2 and Hippo pathway and is not influenced by alteration of actin fibers. Deletion of Thbs1 in mice inhibited Thbs1/integrin β1/YAP signaling, leading to maladaptive remodeling of the aorta in response to pressure overload and inhibition of neointima formation upon carotid artery ligation, exerting context-dependent effects on the vessel wall. We thus propose a mechanism of matrix mechanotransduction centered on Thbs1, connecting mechanical stimuli to YAP signaling during vascular remodeling in vivo.
Keywords: YAP; extracellular matrix (ECM); mechanotransduction; thrombospondin-1; vessel remodeling.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
RAP2 mediates mechanoresponses of the Hippo pathway.Nature. 2018 Aug;560(7720):655-660. doi: 10.1038/s41586-018-0444-0. Epub 2018 Aug 22. Nature. 2018. PMID: 30135582 Free PMC article.
-
Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer.J Exp Clin Cancer Res. 2018 Jul 28;37(1):175. doi: 10.1186/s13046-018-0850-z. J Exp Clin Cancer Res. 2018. PMID: 30055645 Free PMC article.
-
Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway.Cell Rep. 2017 Mar 7;18(10):2464-2479. doi: 10.1016/j.celrep.2017.02.041. Cell Rep. 2017. PMID: 28273460
-
Control of cellular responses to mechanical cues through YAP/TAZ regulation.J Biol Chem. 2019 Nov 15;294(46):17693-17706. doi: 10.1074/jbc.REV119.007963. Epub 2019 Oct 8. J Biol Chem. 2019. PMID: 31594864 Free PMC article. Review.
-
Role of extracellular matrix and YAP/TAZ in cell fate determination.Cell Signal. 2014 Feb;26(2):186-91. doi: 10.1016/j.cellsig.2013.11.006. Epub 2013 Nov 9. Cell Signal. 2014. PMID: 24216612 Review.
Cited by
-
Targeting integrin pathways: mechanisms and advances in therapy.Signal Transduct Target Ther. 2023 Jan 2;8(1):1. doi: 10.1038/s41392-022-01259-6. Signal Transduct Target Ther. 2023. PMID: 36588107 Free PMC article. Review.
-
Targeting Mechanosensitive Piezo1 Alleviated Renal Fibrosis Through p38MAPK-YAP Pathway.Front Cell Dev Biol. 2021 Nov 5;9:741060. doi: 10.3389/fcell.2021.741060. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34805150 Free PMC article.
-
Pathogenic mechanisms and therapeutic implications of extracellular matrix remodelling in cerebral vasospasm.Fluids Barriers CNS. 2023 Nov 4;20(1):81. doi: 10.1186/s12987-023-00483-8. Fluids Barriers CNS. 2023. PMID: 37925414 Free PMC article. Review.
-
ADAM (a Disintegrin and Metalloproteinase) 15 Deficiency Exacerbates Ang II (Angiotensin II)-Induced Aortic Remodeling Leading to Abdominal Aortic Aneurysm.Arterioscler Thromb Vasc Biol. 2020 Aug;40(8):1918-1934. doi: 10.1161/ATVBAHA.120.314600. Epub 2020 Jun 11. Arterioscler Thromb Vasc Biol. 2020. PMID: 32522006 Free PMC article.
-
Single-nucleus analysis of thoracic perivascular adipose tissue reveals critical changes in cell composition, communication, and gene regulatory networks induced by a high fat hypertensive diet.bioRxiv [Preprint]. 2025 Feb 14:2025.02.13.636878. doi: 10.1101/2025.02.13.636878. bioRxiv. 2025. PMID: 39990347 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

