Diverse Allyl Glucosinolate Catabolites Independently Influence Root Growth and Development
- PMID: 32321840
- PMCID: PMC7333702
- DOI: 10.1104/pp.20.00170
Diverse Allyl Glucosinolate Catabolites Independently Influence Root Growth and Development
Abstract
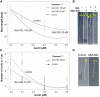
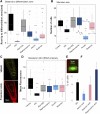
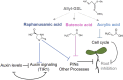
Glucosinolates (GSLs) are sulfur-containing defense metabolites produced in the Brassicales, including the model plant Arabidopsis (Arabidopsis thaliana). Previous work suggests that specific GSLs may function as signals to provide direct feedback regulation within the plant to calibrate defense and growth. These GSLs include allyl-GSL, a defense metabolite that is one of the most widespread GSLs in Brassicaceae and has also been associated with growth inhibition. Here we show that at least three separate potential catabolic products of allyl-GSL or closely related compounds affect growth and development by altering different mechanisms influencing plant development. Two of the catabolites, raphanusamic acid and 3-butenoic acid, differentially affect processes downstream of the auxin signaling cascade. Another catabolite, acrylic acid, affects meristem development by influencing the progression of the cell cycle. These independent signaling events propagated by the different catabolites enable the plant to execute a specific response that is optimal to any given environment.
© 2020 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Agrawal AA, Strauss SY, Stout MJ(1999) Costs of induced responses and tolerance to herbivory in male and female fitness components of wild radish. Evolution 53: 1093–1104 - PubMed
-
- Bartling D, Seedorf M, Mithöfer A, Weiler EW(1992) Cloning and expression of an Arabidopsis nitrilase which can convert indole-3-acetonitrile to the plant hormone, indole-3-acetic acid. Eur J Biochem 205: 417–424 - PubMed
-
- Barton KE, Koricheva J(2010) The ontogeny of plant defense and herbivory: Characterizing general patterns using meta-analysis. Am Nat 175: 481–493 - PubMed
-
- Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, et al. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

