Development of a thermophilic coculture for corn fiber conversion to ethanol
- PMID: 32321909
- PMCID: PMC7176698
- DOI: 10.1038/s41467-020-15704-z
Development of a thermophilic coculture for corn fiber conversion to ethanol
Abstract
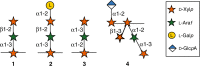
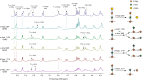
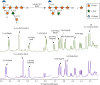
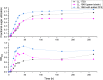
The fiber in corn kernels, currently unutilized in the corn to ethanol process, represents an opportunity for introduction of cellulose conversion technology. We report here that Clostridium thermocellum can solubilize over 90% of the carbohydrate in autoclaved corn fiber, including its hemicellulose component glucuronoarabinoxylan (GAX). However, Thermoanaerobacterium thermosaccharolyticum or several other described hemicellulose-fermenting thermophilic bacteria can only partially utilize this GAX. We describe the isolation of a previously undescribed organism, Herbinix spp. strain LL1355, from a thermophilic microbiome that can consume 85% of the recalcitrant GAX. We sequence its genome, and based on structural analysis of the GAX, identify six enzymes that hydrolyze GAX linkages. Combinations of up to four enzymes are successfully expressed in T. thermosaccharolyticum. Supplementation with these enzymes allows T. thermosaccharolyticum to consume 78% of the GAX compared to 53% by the parent strain and increases ethanol yield from corn fiber by 24%.
Conflict of interest statement
L.R.L. and C.D.H. receive funding from Enchi Corporation. L.R.L. has personal financial interest in Enchi Corporation, which seeks to commercialize technology related to
Figures

References
-
- Li X, Chen S, Huang H, Jin M. In-situ corn fiber conversion improves ethanol yield in corn dry-mill process. Ind. Crops Prod. 2018;113:217–224. doi: 10.1016/j.indcrop.2018.01.037. - DOI
-
- Kurambhatti C, et al. Ethanol production from corn fiber separated after liquefaction in the dry grind process. Energies. 2018;11:2921. doi: 10.3390/en11112921. - DOI
-
- Grohmann K, Bothast RJ. Saccharification of corn fibre by combined treatment with dilute sulphuric acid and enzymes. Process Biochem. 1997;32:405–415. doi: 10.1016/S0032-9592(96)00095-7. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

