A non-anhydrous, minimally basic protocol for the simplification of nucleophilic 18F-fluorination chemistry
- PMID: 32321927
- PMCID: PMC7176689
- DOI: 10.1038/s41598-020-61845-y
A non-anhydrous, minimally basic protocol for the simplification of nucleophilic 18F-fluorination chemistry
Abstract
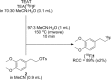
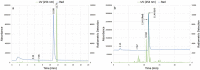
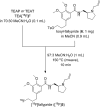
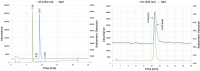
Fluorine-18 radiolabeling typically includes several conserved steps including elution of the [18F]fluoride from an anion exchange cartridge with a basic solution of K2CO3 or KHCO3 and Kryptofix 2.2.2. in mixture of acetonitrile and water followed by rigorous azeotropic drying to remove the water. In this work we describe an alternative "non-anhydrous, minimally basic" ("NAMB") technique that simplifies the process and avoids the basic conditions that can sometimes limit the scope and efficiency of [18F]fluoride incorporation chemistry. In this approach, [18F]F- is eluted from small (10-12 mg) anion-exchange cartridges with solutions of tetraethylammonium bicarbonate, perchlorate or tosylate in polar aprotic solvents containing 10-50% water. After dilution with additional aprotic solvent, these solutions are used directly in nucleophilic aromatic and aliphatic 18F-fluorination reactions, obviating the need for azeotropic drying. Perchlorate and tosylate are minimally basic anions that are nevertheless suitable for removal of [18F]F- from the anion-exchange cartridge. As proof-of-principle, "NAMB" chemistry was utilized for the synthesis of the dopamine D2/D3 antagonist [18F]fallypride.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Cryptate mediated nucleophilic 18F-fluorination without azeotropic drying.Nuklearmedizin. 2012;51(1):1-8. doi: 10.3413/Nukmed-0425-11-08. Epub 2011 Oct 11. Nuklearmedizin. 2012. PMID: 21989864
-
18F-Fluorination Using Tri-Tert-Butanol Ammonium Iodide as Phase-Transfer Catalyst: An Alternative Minimalist Approach.Pharmaceuticals (Basel). 2021 Aug 24;14(9):833. doi: 10.3390/ph14090833. Pharmaceuticals (Basel). 2021. PMID: 34577533 Free PMC article.
-
High-efficiency [18F]fluoride pre-concentration using a laser-micromachined anion-exchange micro-cartridge.EJNMMI Radiopharm Chem. 2025 Mar 21;10(1):11. doi: 10.1186/s41181-025-00334-x. EJNMMI Radiopharm Chem. 2025. PMID: 40113734 Free PMC article.
-
Isotope Exchange-Based 18F-Labeling Methods.Bioconjug Chem. 2023 Jan 18;34(1):140-161. doi: 10.1021/acs.bioconjchem.2c00530. Epub 2023 Jan 3. Bioconjug Chem. 2023. PMID: 36594786 Review.
-
Electrochemical Radiofluorination of Small Molecules: New Advances.Chem Rec. 2021 Sep;21(9):2397-2410. doi: 10.1002/tcr.202100086. Epub 2021 May 19. Chem Rec. 2021. PMID: 34010479 Review.
Cited by
-
Highlight selection of radiochemistry and radiopharmacy developments by editorial board (January-June 2020).EJNMMI Radiopharm Chem. 2021 Jan 28;6(1):5. doi: 10.1186/s41181-020-00118-5. EJNMMI Radiopharm Chem. 2021. PMID: 33507426 Free PMC article. Review.
-
Detrimental impact of aqueous mobile phases on 18F-labelled radiopharmaceutical analysis via radio-TLC.Anal Methods. 2023 Jan 19;15(3):377-387. doi: 10.1039/d2ay01206e. Anal Methods. 2023. PMID: 36542448 Free PMC article.
-
[18F]fluoride Activation and 18F-Labelling in Hydrous Conditions-Towards a Microfluidic Synthesis of PET Radiopharmaceuticals.Molecules. 2023 Dec 26;29(1):147. doi: 10.3390/molecules29010147. Molecules. 2023. PMID: 38202730 Free PMC article.
-
Automation of Copper-Mediated 18F-Fluorination of Aryl Pinacol Boronates Using 4-Dimethylaminopyridinium Triflate.Molecules. 2024 Jul 16;29(14):3342. doi: 10.3390/molecules29143342. Molecules. 2024. PMID: 39064920 Free PMC article.
-
A rapid and systematic approach for the optimization of radio thin-layer chromatography resolution.J Chromatogr A. 2023 Jan 4;1687:463656. doi: 10.1016/j.chroma.2022.463656. Epub 2022 Nov 22. J Chromatogr A. 2023. PMID: 36463649 Free PMC article.
References
-
- Hamacher K, Coenen H, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-[F-18]-fluoro-2-deoxy-d-glucose using aminopolyether supported nucleophilic-substitution. J. Nucl. Med. 1986;27:235–238. - PubMed
-
- Kilbourn, M. R. & Huizenga, J. R. Fluorine-18 Labeling of Radiopharmaceuticals (National Academy Press, 1990).
-
- Aerts J, et al. Fast production of highly concentrated reactive F-18 fluoride for aliphatic and aromatic nucleophilic radiolabelling. Tetrahedron Lett. 2010;51:64–66. doi: 10.1016/j.tetlet.2009.10.085. - DOI
-
- Perrio C, et al. [18F]-Fluoride capture and release: azeotropic drying free nucleophilic aromatic radiofluorination assisted by a phosphonium borane. ChemComm. 2017;53:340–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

