Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer's disease
- PMID: 32321934
- PMCID: PMC7176648
- DOI: 10.1038/s41387-020-0115-8
Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer's disease
Abstract
Objective: Alzheimer's disease (AD) is a global health problem without effective methods to alleviate the disease progression. Amyloid β-protein (Aβ) is widely accepted as a key biomarker for AD. Metabolic syndromes, including obesity and insulin resistance, are key high risk factors for AD. Akkermansia muciniphila (Akk), the only representative human gut microbe in the genus Verrucomicrobia, can prevent the weight gain caused by a high-fat diet, repair the damaged integrity of the intestinal epithelium barrier, reduce endotoxin levels in blood and improve insulin resistance. The aim of this study is to explore the impact of Akk administration in AD model mice in different diets.
Methods: APP/PS1 mice were fed either a normal chow diet or a high-fat diet and were treated with Akk by gavage each day for 6 months. The impacts of Akk on glucose metabolism, intestinal barrier and lipid metabolism in the mouse model of AD were determined. Changes in brain pathology and neuroethology were also analyzed.
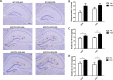
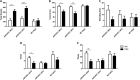
Results: Akk effectively reduced the fasting blood glucose and serum diamine oxidase levels, and alleviated the reduction of colonic mucus cells in APP/PS1 mice. After treatment with Akk, the APP/PS1 mice showed obviously reduced blood lipid levels, improved hepatic steatosis and scapular brown fat whitening. Moreover, Akk promoted the reduction of Aβ 40-42 levels in the cerebral cortex of APP/PS1 mice, shortened the study time and improved the completion rate in Y-maze tests.
Conclusion: Akk effectively improved glucose tolerance, intestine barrier dysfunction and dyslipidemia in AD model mice. Our study results suggested that Akk could delay the pathological changes in the brain and relieve impairment of spatial learning and memory in AD model mice, which provides a new strategy for prevention and treatment of AD.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Ameliorative effects of Akkermansia muciniphila on anxiety-like behavior and cognitive deficits in a rat model of Alzheimer's disease.Brain Res. 2024 Dec 15;1845:149280. doi: 10.1016/j.brainres.2024.149280. Epub 2024 Oct 16. Brain Res. 2024. PMID: 39419309
-
Human gut microbiota Agathobaculum butyriciproducens improves cognitive impairment in LPS-induced and APP/PS1 mouse models of Alzheimer's disease.Nutr Res. 2021 Feb;86:96-108. doi: 10.1016/j.nutres.2020.12.010. Epub 2020 Dec 9. Nutr Res. 2021. PMID: 33551257
-
Cyanidin 3-O-β-glucopyranoside activates peroxisome proliferator-activated receptor-γ and alleviates cognitive impairment in the APP(swe)/PS1(ΔE9) mouse model.Biochim Biophys Acta. 2016 Sep;1862(9):1786-800. doi: 10.1016/j.bbadis.2016.05.016. Epub 2016 May 27. Biochim Biophys Acta. 2016. PMID: 27240542
-
Protective Effects of Bifidobacterium Breve MCC1274 as a Novel Therapy for Alzheimer's Disease.Nutrients. 2025 Jan 31;17(3):558. doi: 10.3390/nu17030558. Nutrients. 2025. PMID: 39940416 Free PMC article. Review.
-
High-Fat Diets in Animal Models of Alzheimer's Disease: How Can Eating Too Much Fat Increase Alzheimer's Disease Risk?J Alzheimers Dis. 2024;97(3):977-1005. doi: 10.3233/JAD-230118. J Alzheimers Dis. 2024. PMID: 38217592 Free PMC article. Review.
Cited by
-
Convergent pathways of the gut microbiota-brain axis and neurodegenerative disorders.Gastroenterol Rep (Oxf). 2022 May 16;10:goac017. doi: 10.1093/gastro/goac017. eCollection 2022. Gastroenterol Rep (Oxf). 2022. PMID: 35582476 Free PMC article. Review.
-
Probiotics as potential therapeutic options for Alzheimer's disease.Appl Microbiol Biotechnol. 2021 Oct;105(20):7721-7730. doi: 10.1007/s00253-021-11607-1. Epub 2021 Oct 1. Appl Microbiol Biotechnol. 2021. PMID: 34596721 Review.
-
Health and Disease: Akkermansia muciniphila, the Shining Star of the Gut Flora.Research (Wash D C). 2023;6:0107. doi: 10.34133/research.0107. Epub 2023 Apr 3. Research (Wash D C). 2023. PMID: 37040299 Free PMC article.
-
Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283.EFSA J. 2021 Sep 1;19(9):e06780. doi: 10.2903/j.efsa.2021.6780. eCollection 2021 Sep. EFSA J. 2021. PMID: 34484452 Free PMC article.
-
Therapeutic Prospect of New Probiotics in Neurodegenerative Diseases.Microorganisms. 2023 Jun 8;11(6):1527. doi: 10.3390/microorganisms11061527. Microorganisms. 2023. PMID: 37375029 Free PMC article. Review.
References
-
- Kuhlmann J, Andreasson U, Pannee J. CSF Aá1-42: an excellent but complicated Alzheimer's biomarker − a route to standardisation. Clin. Chim. Acta. 2016;467:27–33. - PubMed
-
- Galimberti D, Scarpini E. Progress in Alzheimer’s disease. J. Neurol. 2012;259:201–211. - PubMed
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. - PubMed
-
- Campos-Pena V, et al. Metabolic syndrome as a risk factor for alzheimer’s disease: is abeta a crucial factor in both pathologies? Antioxid. Redox Signal. 2017;26:542–560. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

