Computer-aided synthesis of dapsone-phytochemical conjugates against dapsone-resistant Mycobacterium leprae
- PMID: 32322091
- PMCID: PMC7176699
- DOI: 10.1038/s41598-020-63913-9
Computer-aided synthesis of dapsone-phytochemical conjugates against dapsone-resistant Mycobacterium leprae
Abstract
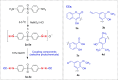
Leprosy continues to be the belligerent public health hazard for the causation of high disability and eventual morbidity cases with stable prevalence rates, even with treatment by the on-going multidrug therapy (MDT). Today, dapsone (DDS) resistance has led to fear of leprosy in more unfortunate people of certain developing countries. Herein, DDS was chemically conjugated with five phytochemicals independently as dapsone-phytochemical conjugates (DPCs) based on azo-coupling reaction. Possible biological activities were verified with computational chemistry and quantum mechanics by molecular dynamics simulation program before chemical synthesis and spectral characterizations viz., proton-HNMR, FTIR, UV and LC-MS. The in vivo antileprosy activity was monitored using the 'mouse-foot-pad propagation method', with WHO recommended concentration 0.01% mg/kg each DPC for 12 weeks, and the host-toxicity testing of the active DPC4 was seen in cultured-human-lymphocytes in vitro. One-log bacilli cells in DDS-resistant infected mice footpads decreased by the DPC4, and no bacilli were found in the DDS-sensitive mice hind pads. Additionally, the in vitro host toxicity study also confirmed that the DCP4 up to 5,000 mg/L level was safety for oral administration, since a minor number of dead cells were found in red color under a fluorescent microscope. Several advanced bioinformatics tools could help locate the potential chemical entity, thereby reducing the time and resources required for in vitro and in vitro tests. DPC4 could be used in place of DDS in MDT, evidenced from in vivo antileprosy activity and in vitro host toxicity study.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- World Health Organization Weekly Epidemiological Record. 2016;92:501–20.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

