Comparison of Clinically Relevant Oncolytic Virus Platforms for Enhancing T Cell Therapy of Solid Tumors
- PMID: 32322662
- PMCID: PMC7163046
- DOI: 10.1016/j.omto.2020.03.003
Comparison of Clinically Relevant Oncolytic Virus Platforms for Enhancing T Cell Therapy of Solid Tumors
Abstract
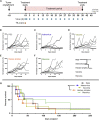
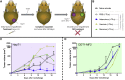
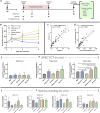
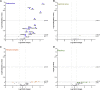
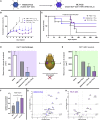
Despite some promising results, the majority of patients do not benefit from T cell therapies, as tumors prevent T cells from entering the tumor, shut down their activity, or downregulate key antigens. Due to their nature and mechanism of action, oncolytic viruses have features that can help overcome many of the barriers currently facing T cell therapies of solid tumors. This study aims to understand how four different oncolytic viruses (adenovirus, vaccinia virus, herpes simplex virus, and reovirus) perform in that task. For that purpose, an immunocompetent in vivo tumor model featuring adoptive tumor-infiltrating lymphocyte (TIL) therapy was used. Tumor growth control (p < 0.001) and survival analyses suggest that adenovirus was most effective in enabling T cell therapy. The complete response rate was 62% for TILs + adenovirus versus 17.5% for TILs + PBS. Of note, TIL biodistribution did not explain efficacy differences between viruses. Instead, immunostimulatory shifts in the tumor microenvironment mirrored efficacy results. Overall, the use of oncolytic viruses can improve the utility of T cell therapies, and additional virus engineering by arming with transgenes can provide further antitumor effects. This phenomenon was seen when an unarmed oncolytic adenovirus was compared to Ad5/3-E2F-d24-hTNFa-IRES-hIL2 (TILT-123). A clinical trial is ongoing, where patients receiving TIL treatment also receive TILT-123 (ClinicalTrials.gov: NCT04217473).
Keywords: T cell therapy; adenovirus; gene therapy; herpes simplex virus; immunotherapy; oncolytic virus; reovirus; solid tumor; tumor microenvironment; vaccinia virus.
© 2020 The Authors.
Figures

References
-
- Kelly E., Russell S.J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. - PubMed
-
- Pikor L.A., Bell J.C., Diallo J.S. Oncolytic Viruses: Exploiting Cancer’s Deal with the Devil. Trends Cancer. 2015;1:266–277. - PubMed
-
- Hoster H.A., Zanes R.P., Jr., Von Haam E. Studies in Hodgkin’s syndrome; the association of viral hepatitis and Hodgkin’s disease; a preliminary report. Cancer Res. 1949;9:473–480. - PubMed
-
- Huebner R.J., Rowe W.P., Schatten W.E., Smith R.R., Thomas L.B. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–1218. - PubMed
-
- Southam C.M., Moore A.E. Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer. 1952;5:1025–1034. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

