Pharmacokinetics and Pharmacodynamics of Posaconazole
- PMID: 32323222
- PMCID: PMC7183491
- DOI: 10.1007/s40265-020-01306-y
Pharmacokinetics and Pharmacodynamics of Posaconazole
Abstract
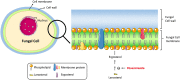
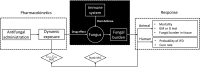
Posaconazole is typically used for preventing invasive yeast and mold infections such as invasive aspergillosis in high-risk immunocompromised patients. The oral suspension was the first released formulation and many pharmacokinetic and pharmacodynamic studies of this formulation have been published. Erratic absorption profiles associated with this formulation were widely reported. Posaconazole exposure was found to be significantly influenced by food and many gastrointestinal conditions, including pH and motility. As a result, low posaconazole plasma concentrations were obtained in large groups of patients. These issues of erratic absorption urged the development of the subsequently marketed delayed-release tablet, which proved to be associated with higher and more stable exposure profiles. Shortly thereafter, an intravenous formulation was released for patients who are not able to take oral formulations. Both new formulations require a loading dose on day 1 to achieve high posaconazole concentrations more quickly, which was not possible with the oral suspension. So far, there appears to be no evidence of increased toxicity correlated to the higher posaconazole exposure achieved with the regimen for these formulations. The higher systemic availability of posaconazole for the delayed-release tablet and intravenous formulation have resulted in these two formulations being preferable for both prophylaxis and treatment of invasive fungal disease. This review aimed to integrate the current knowledge on posaconazole pharmacokinetics, pharmacodynamics, major toxicity, existing resistance, clinical experience in special populations, and new therapeutic strategies in order to get a clear understanding of the clinical use of this drug.
Conflict of interest statement
No disclosures are applicable for this work. Disclosures outside of this work: R.J.B. and P.V have served as consultants to Astellas Pharma, Inc., F2G, Amplyx, Gilead Sciences, Merck Sharp & Dohme Corp., and Pfizer, Inc., and have received unrestricted and research grants from Astellas Pharma, Inc., Gilead Sciences, Merck Sharp & Dohme Corp., and Pfizer, Inc. All contracts were through Radboudumc, and all payments were invoiced by Radboudumc. None of the other authors have a conflict to declare.
Figures
References
-
- U.S FDA. Noxafil instruction. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205053s1lbl.pdf. Accessed 15 Apr 2020.
-
- European Medicines Agency. Summary of posaconazole characteristics. 2010. https://www.ema.europa.eu/en/documents/product-information/noxafil-epar-.... Accessed 15 Apr 2020.
-
- The European Committee on Antimicrobial Susceptibility Testing. Posaconazole: Rationale for the EUCAST clinical breakpoints, version 2.0. 2017. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/.... Accessed 15 Apr 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

