Contribution of mixed pathology to medial temporal lobe atrophy in Alzheimer's disease
- PMID: 32323446
- PMCID: PMC7715004
- DOI: 10.1002/alz.12079
Contribution of mixed pathology to medial temporal lobe atrophy in Alzheimer's disease
Abstract
Introduction: It is unclear how different proteinopathies (tau, transactive response DNA-binding protein 43 [TDP-43], amyloid β [Aβ], and α-synuclein) contribute to atrophy within medial temporal lobe (MTL) subregions in Alzheimer's disease (AD).
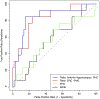
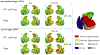
Methods: We utilized antemortem structural magnetic resonance imaging (MRI) data to measure MTL substructures and examined the relative contribution of tau, TDP-43, Aβ, and α-synuclein measured in post-mortem tissue from 92 individuals with intermediate to high AD neuropathology. Receiver-operating characteristic (ROC) curves were analyzed for each subregion in order to discriminate TDP-43-negative and TDP-43-positive patients.
Results: TDP-43 was strongly associated with anterior MTL regions, whereas tau was relatively more associated with the posterior hippocampus. Among the MTL regions, the anterior hippocampus showed the highest area under the ROC curve (AUC).
Discussion: We found specific contributions of different pathologies on MTL substructure in this population with AD neuropathology. The anterior hippocampus may be a relevant region to detect concomitant TDP-43 pathology in the MTL of patients with AD.
Keywords: Alzheimer's disease; TDP-43; atrophy; medial temporal lobe; neuropathology; tau.
© 2020 the Alzheimer's Association.
Conflict of interest statement
DISCLOSURE/CONFLICTS OF INTEREST
David A. Wolk received consultation fees from Eli Lilly, Janssen, and Merck. David A. Wolk receives grant support from Avid Radiopharmaceuticals/Eli Lilly, Biogen, Functional Neuromodulation, and Merck. John Q. Trojanowski has received research support from Eli Lilly. John Q. Trojanowski may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is co-inventor, and he received revenue from the sale of Avid to Eli Lilly as co-inventor on imaging-related patents submitted by the University of Pennsylvania. Corey T. McMillan receives research funding from Biogen, Inc, and provides consulting services for Invicro and Axon Advisors on behalf of Translational Bioinformatics, LLC. He also receives an honorarium as Associate Editor of NeuroImage: Clinical. Long Xie received personal consulting fees from Galileo CDS, Inc.
Figures


References
-
- Pini L, Pievani M, Bocchetta M, et al. Brain atrophy in Alzheimer’s Disease and aging. Ageing Res Rev. 2016;30:1–24. - PubMed
-
- Tondelli M, Wilcock GK, Nichelli P, De Jager CA, Jenkinson M, Zamboni G. Structural MRI changes detectable up to ten years before clinical Alzheimer’s disease. Neurobiol Aging. 2012;33(4): 825.e25–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

