Pigment epithelium‑derived factor facilitates NLRP3 inflammasome activation through downregulating cytidine monophosphate kinase 2: A potential treatment strategy for missed abortion
- PMID: 32323732
- PMCID: PMC7138263
- DOI: 10.3892/ijmm.2020.4517
Pigment epithelium‑derived factor facilitates NLRP3 inflammasome activation through downregulating cytidine monophosphate kinase 2: A potential treatment strategy for missed abortion
Abstract
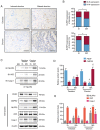
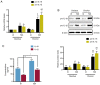
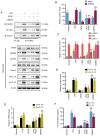
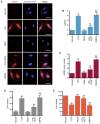
A number of conditions may underlie the occurrence of missed abortion (MA), including inflammation. Pigment epithelium‑derived factor (PEDF) is a novel mediator of the inflammation‑related nucleotide‑binding oligomerization domain‑like receptor protein 3 (NLRP3) inflammasome, which is associated with several human diseases. However, the association between MA and NLRP3 inflammasome, and whether PEDF is reduced in MA, remain unknown. In the present study, the decidua and chorion tissues of patients who had suffered a MA were examined, and a lipopolysaccharide (LPS)‑induced human chorionic trophoblast HTR8/SVneo cell model was established to mimic MA in vitro. The results revealed that cytidine monophosphate kinase 2 (CMPK2) expression and NLRP3 inflammasome activation, downstream pro‑IL‑18 and pro‑IL‑1β expression, and IL‑18 and IL‑1β release, were all significantly increased in MA tissues or LPS‑induced HTR8/SVneo cells. PEDF reversed the increase in CMPK2 expression and activation of the NLRP3 inflammasome axis and, thus, downregulated the production of mitochondrial reactive oxygen species and mitochondrial DNA release, resulting in reduced lactate dehydrogenase release, and a resultant decrease in cell viability. Recovery of CMPK2 expression abolished all the effects of PEDF, indicating that CMPK2 may be an effector downstream of PEDF. PEDF reduced CMPK2 protein levels but did not affect the mRNA levels, and treatment with the proteasomal inhibitor MG132 significantly reversed this reduction in CMPK2 protein levels. Furthermore, a ubiquitination assay of immunoprecipitation demonstrated that CMPK2 was polyubiquitinated in the presence of LPS, PEDF and MG132. These results indicated that the NLRP3 inflammasome is implicated in the pathogenesis of MA, and PEDF may reduce MA through ubiquitin‑dependent proteasomal degradation of CMPK2 to inhibit NLRP3 activation, which may serve as a novel strategy for preventing or reducing the risk of MA.
Figures
Similar articles
-
PEDF Inhibits the Activation of NLRP3 Inflammasome in Hypoxia Cardiomyocytes through PEDF Receptor/Phospholipase A2.Int J Mol Sci. 2016 Dec 12;17(12):2064. doi: 10.3390/ijms17122064. Int J Mol Sci. 2016. PMID: 27973457 Free PMC article.
-
CMPK2 promotes NLRP3 inflammasome activation via mtDNA-STING pathway in house dust mite-induced allergic rhinitis.Clin Transl Med. 2025 Jan;15(1):e70180. doi: 10.1002/ctm2.70180. Clin Transl Med. 2025. PMID: 39799434 Free PMC article.
-
MiR-494-3p alleviates acute lung injury through regulating NLRP3 activation by targeting CMPK2.Biochem Cell Biol. 2021 Jun;99(3):286-295. doi: 10.1139/bcb-2020-0243. Epub 2021 May 26. Biochem Cell Biol. 2021. PMID: 34037470
-
Mechanism and Regulation of NLRP3 Inflammasome Activation.Trends Biochem Sci. 2016 Dec;41(12):1012-1021. doi: 10.1016/j.tibs.2016.09.002. Epub 2016 Sep 23. Trends Biochem Sci. 2016. PMID: 27669650 Free PMC article. Review.
-
Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance.Arch Pharm Res. 2016 Nov;39(11):1503-1518. doi: 10.1007/s12272-016-0827-4. Epub 2016 Sep 7. Arch Pharm Res. 2016. PMID: 27600432 Review.
Cited by
-
The role of pyroptosis in the occurrence and development of pregnancy-related diseases.Front Immunol. 2024 Sep 16;15:1400977. doi: 10.3389/fimmu.2024.1400977. eCollection 2024. Front Immunol. 2024. PMID: 39351226 Free PMC article. Review.
-
Electroacupuncture Inhibits NLRP3 Activation by Regulating CMPK2 After Spinal Cord Injury.Front Immunol. 2022 Mar 24;13:788556. doi: 10.3389/fimmu.2022.788556. eCollection 2022. Front Immunol. 2022. PMID: 35401582 Free PMC article.
-
Clinical Efficacy and Safety Study of Mifepristone with Misoprostol Treatment in Patients with Missed Abortion.Evid Based Complement Alternat Med. 2021 Sep 28;2021:9983023. doi: 10.1155/2021/9983023. eCollection 2021. Evid Based Complement Alternat Med. 2021. Retraction in: Evid Based Complement Alternat Med. 2023 Jun 21;2023:9851738. doi: 10.1155/2023/9851738. PMID: 34621327 Free PMC article. Retracted.
-
Knockdown of DAPK1 inhibits IL-1β-induced inflammation and cartilage degradation in human chondrocytes by modulating the PEDF-mediated NF-κB and NLRP3 inflammasome pathway.Innate Immun. 2024 Jan;30(1):21-30. doi: 10.1177/17534259221086837. Epub 2022 Nov 21. Innate Immun. 2024. PMID: 36412004 Free PMC article.
-
Mitochondrial dysfunction in pregnancy loss: a review.Mol Cell Biochem. 2025 May;480(5):2749-2764. doi: 10.1007/s11010-024-05171-1. Epub 2024 Dec 2. Mol Cell Biochem. 2025. PMID: 39621222 Review.
References
-
- Wood SL, Brain PH. Medical management of missed abortion: A randomized clinical trial. Obstet Gynecol. 2002;99:563–566. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

