Long non‑coding RNA NORAD regulates angiogenesis of human umbilical vein endothelial cells via miR‑590‑3p under hypoxic conditions
- PMID: 32323787
- PMCID: PMC7185274
- DOI: 10.3892/mmr.2020.11064
Long non‑coding RNA NORAD regulates angiogenesis of human umbilical vein endothelial cells via miR‑590‑3p under hypoxic conditions
Abstract
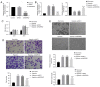
Dysregulation of angiogenesis can be caused by hypoxia, which may result in severe diseases of the heart, including coronary artery disease. Hypoxia‑inducible factor 1 (HIF‑1) modulates angiogenesis via the regulation of several angiogenic factors. However, the underlying mechanism of hypoxia‑induced angiogenesis remains unknown. In the present study, it was hypothesized that long non‑coding RNA (lncRNA) non‑coding RNA activated by DNA damage (NORAD) may serve a role in the process of angiogenesis via the regulation of microRNA(miR)‑590‑3p under hypoxic conditions. The effect of NORAD and miR‑590‑3p on cell viability and properties associated with angiogenesis, including cell migration and tube formation in human umbilical vein endothelial cells (HUVECs) under hypoxic conditions, were assessed. Potential downstream angiogenic factors of miR‑590‑3p were also determined by molecular experiments. It was identified that NORAD expression was upregulated and miR‑590‑3p expression was downregulated in hypoxia‑exposed HUVECs, and also in myocardial infarction (MI) left ventricle tissues in mice. Moreover, downregulation of NORAD expression resulted in decreased cell viability and angiogenic capacity, but further knocking down miR‑590‑3p expression reversed these alterations, resulting in increased cell migration and tube formation in HUVECs under hypoxic conditions for 24 h. It was demonstrated that NORAD overexpression also increased cell vitality and tube‑formation capacity. Furthermore, NORAD was identified to bind with miR‑590‑3p directly, and miR‑590‑3p was shown to target certain proangiogenic agents, such as vascular endothelial growth factor (VEGF)A, fibroblast growth factor (FGF)1 and FGF2 directly. Therefore, the present results suggested that lncRNA NORAD may bind with miR‑590‑3p to regulate the angiogenic ability of HUVECs via the regulation of several downstream proangiogenic factors under hypoxia. Thus, the lncRNA NORAD/miR‑590‑3p axis may be a novel regulatory pathway in the angiogenic mechanisms in HUVECs, which highlights a potentially novel perspective for treating ischemia/hypoxia‑induced angiogenic diseases.
Keywords: hypoxia; non-coding rna activated by dna damage; transcriptional activation; microrna-590-3p.
Figures






References
-
- Ferrara N. Vascular endothelial growth factor: Molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. - PubMed

