Systematic age-, organ-, and diet-associated ionome remodeling and the development of ionomic aging clocks
- PMID: 32323920
- PMCID: PMC7253066
- DOI: 10.1111/acel.13119
Systematic age-, organ-, and diet-associated ionome remodeling and the development of ionomic aging clocks
Abstract
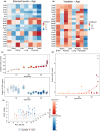
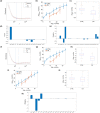
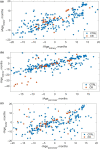
Aging involves coordinated yet distinct changes in organs and systems throughout life, including changes in essential trace elements. However, how aging affects tissue element composition (ionome) and how these changes lead to dysfunction and disease remain unclear. Here, we quantified changes in the ionome across eight organs and 16 age groups of mice. This global profiling revealed novel interactions between elements at the level of tissue, age, and diet, and allowed us to achieve a broader, organismal view of the aging process. We found that while the entire ionome steadily transitions along the young-to-old trajectory, individual organs are characterized by distinct element changes. The ionome of mice on calorie restriction (CR) moved along a similar but shifted trajectory, pointing that at the organismal level this dietary regimen changes metabolism in order to slow down aging. However, in some tissues CR mimicked a younger state of control mice. Even though some elements changed with age differently in different tissues, in general aging was characterized by the reduced levels of elements as well as their increased variance. The dataset we prepared also allowed to develop organ-specific, ionome-based markers of aging that could help monitor the rate of aging. In some tissues, these markers reported the lifespan-extending effect of CR. These aging biomarkers have the potential to become an accessible tool to test the age-modulating effects of interventions.
Keywords: aging; biomarkers of aging; calorie restriction; chemical elements; ionome.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

