Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial
- PMID: 32325037
- PMCID: PMC7172913
- DOI: 10.1016/S1473-3099(20)30248-6
Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial
Abstract
Background: The Middle East respiratory syndrome coronavirus (MERS-CoV) causes a respiratory disease with a case fatality rate of up to 35%. Given its potential to cause a public health emergency and the absence of efficacious drugs or vaccines, MERS is one of the WHO priority diseases warranting urgent research and development of countermeasures. We aimed to assess safety and tolerability of an anti-MERS-CoV modified vaccinia virus Ankara (MVA)-based vaccine candidate that expresses the MERS-CoV spike glycoprotein, MVA-MERS-S, in healthy adults.
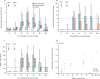
Methods: This open-label, phase 1 trial was done at the University Medical Center Hamburg-Eppendorf (Hamburg, Germany). Participants were healthy men and women aged 18-55 years with no clinically significant health problems as determined during medical history and physical examination, a body-mass index of 18·5-30·0 kg/m2 and weight of more than 50 kg at screening, and a negative pregnancy test for women. A key exclusion criterion was a previous MVA vaccination. For the prime immunisation, participants received doses of 1 × 107 plaque-forming unit (PFU; low-dose group) or 1 × 108 PFU (high-dose group) MVA-MERS-S intramuscularly. A second identical dose was administered intramuscularly as a booster immunisation 28 days after first injection. As a control group for immunogenicity analyses, blood samples were drawn at identical study timepoints from six healthy adults, who did not receive any injections. The primary objectives of the study were safety and tolerability of the two dosage levels and reactogenicity after administration. Immunogenicity was assessed as a secondary endpoint by ELISA and neutralisation tests. T-cell immunity was evaluated by interferon-γ-linked enzyme-linked immune absorbent spot assay. All participants who were vaccinated at least once were included in the safety analysis. Immunogenicity was analysed in the participants who completed 6 months of follow-up. This trial is registered with ClinicalTrials.gov, NCT03615911, and EudraCT, 2014-003195-23 FINDINGS: From Dec 17, 2017, to June 5, 2018, 26 participants (14 in the low-dose group and 12 in the high-dose group) were enrolled and received the first dose of the vaccine according to their group allocation. Of these, 23 participants (12 in the low-dose group and 11 in the high-dose group) received a second dose of MVA-MERS-S according to their group allocation after a 28-day interval and completed follow-up. Homologous prime-boost immunisation with MVA-MERS-S revealed a benign safety profile with only transient mild-to-moderate reactogenicity. Participants had no severe or serious adverse events. 67 vaccine-related adverse events were reported in ten (71%) of 14 participants in the low-dose group, and 111 were reported in ten (83%) of 12 participants in the high-dose group. Solicited local reactions were the most common adverse events: pain was observed in 17 (65%; seven in the low-dose group vs ten in the high-dose group) participants, swelling in ten (38%; two vs eight) participants, and induration in ten (38%; one vs nine) participants. Headaches (observed in seven participants in the low-dose group vs nine in the high-dose group) and fatigue or malaise (ten vs seven participants) were the most common solicited systemic adverse events. All adverse events resolved swiftly (within 1-3 days) and without sequelae. Following booster immunisation, nine (75%) of 12 participants in the low-dose group and 11 (100%) participants in the high-dose group showed seroconversion using a MERS-CoV S1 ELISA at any timepoint during the study. Binding antibody titres correlated with MERS-CoV-specific neutralising antibodies (Spearman's correlation r=0·86 [95% CI 0·6960-0·9427], p=0·0001). MERS-CoV spike-specific T-cell responses were detected in ten (83%) of 12 immunised participants in the low-dose group and ten (91%) of 11 immunised participants in the high-dose group.
Interpretation: Vaccination with MVA-MERS-S had a favourable safety profile without serious or severe adverse events. Homologous prime-boost immunisation induced humoral and cell-mediated responses against MERS-CoV. A dose-effect relationship was demonstrated for reactogenicity, but not for vaccine-induced immune responses. The data presented here support further clinical testing of MVA-MERS-S in larger cohorts to advance MERS vaccine development.
Funding: German Center for Infection Research.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Two Middle East respiratory syndrome vaccines: first step for other coronavirus vaccines?Lancet Infect Dis. 2020 Jul;20(7):760-761. doi: 10.1016/S1473-3099(20)30317-0. Epub 2020 Apr 21. Lancet Infect Dis. 2020. PMID: 32325036 Free PMC article. No abstract available.
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. - PubMed
-
- WHO MERS monthly summary. January 2020. http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html
-
- WHO 2015 MERS outbreak in Republic of Korea. https://www.who.int/westernpacific/emergencies/2015-mers-outbreak
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

