Non-Coding RNAs as Key Regulators of Glutaminolysis in Cancer
- PMID: 32326003
- PMCID: PMC7216265
- DOI: 10.3390/ijms21082872
Non-Coding RNAs as Key Regulators of Glutaminolysis in Cancer
Abstract
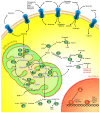
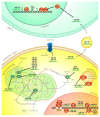
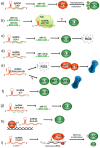
Cancer cells exhibit exacerbated metabolic activity to maintain their accelerated proliferation and microenvironmental adaptation in order to survive under nutrient-deficient conditions. Tumors display an increase in glycolysis, glutaminolysis and fatty acid biosynthesis, which provide their energy source. Glutamine is critical for fundamental cellular processes, where intermediate metabolites produced through glutaminolysis are necessary for the maintenance of mitochondrial metabolism. These include antioxidants to remove reactive oxygen species, and the generation of the nonessential amino acids, purines, pyrimidines and fatty acids required for cellular replication and the activation of cell signaling. Some cancer cells are highly dependent on glutamine consumption since its catabolism provides an anaplerotic pathway to feed the Krebs cycle. Intermediate members of the glutaminolysis pathway have been found to be deregulated in several types of cancers and have been proposed as therapeutic targets and prognostic biomarkers. This review summarizes the main players in the glutaminolysis pathway, how they have been found to be deregulated in cancer and their implications for cancer maintenance. Furthermore, non-coding RNAs are now recognized as new participants in the regulation of glutaminolysis; therefore, their involvement in glutamine metabolism in cancer is discussed in detail.
Keywords: cancer; glutaminolysis; lncRNAs; miRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

