Overexpression of T-bet, GATA-3 and TGF-ß Induces IFN-γ, IL-4/13A, and IL-17A Expression in Atlantic Salmon
- PMID: 32326041
- PMCID: PMC7235720
- DOI: 10.3390/biology9040082
Overexpression of T-bet, GATA-3 and TGF-ß Induces IFN-γ, IL-4/13A, and IL-17A Expression in Atlantic Salmon
Abstract
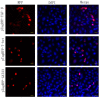
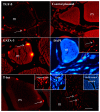
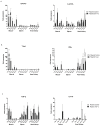
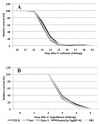
The overexpression of GATA-3, T-bet and TGF-ß may theoretically induce IL-4/A, IFN-γ and IL-17A expression, respectively. Whether this also applies to fish is not yet known. The plasmid vectors encoding reporter gene (RFP)-tagged T-bet, GATA-3 and TGF-β were used as overexpression tools, transfected into cells or injected intramuscularly to monitor the expression of IFN-γ, IL-4/13A and IL-17A. In addition, the fish were either experimentally challenged with Vibrio anguillarum (VA group) or Piscirickettsia salmonis (PS group). The reporter gene (RFP) inserted upstream of the GATA-3, T-bet and TGF-ß genes, was observed in muscle cell nuclei and in inflammatory cells after intramuscular (i.m.) injection. PS group: following the injection of GATA-3 and T-bet-encoding plasmids, the expression of GATA-3 and T-bet was high at the injection site. The spleen expression of IFN-γ, following the injection of a T-bet-encoding plasmid, was significantly higher on day 2. VA group: The T-bet and GATA-3-overexpressing fish expressed high T-bet and GATA-3 mRNA levels in the muscles and on day 4 post-challenge. The expression of TGF-ß in the muscles of fish injected with TGF-ß-encoding plasmids was significantly higher on days 7 (8 days pre-challenge) and 19 (4 days after challenge). The protective effects of the overexpression of T-bet, GATA-3 and TGF-ß on both bacterial infections were negligible.
Keywords: Atlantic salmon; GATA-3; T-bet; TGF-ß; gene expression; overexpression.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

