A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications
- PMID: 32326131
- PMCID: PMC7221918
- DOI: 10.3390/molecules25081909
A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications
Abstract
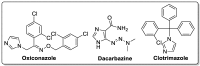
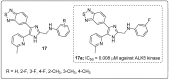
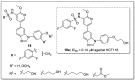
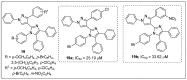
The analogs of nitrogen-based heterocycles occupy an exclusive position as a valuable source of therapeutic agents in medicinal chemistry. More than 75% of drugs approved by the FDA and currently available in the market are nitrogen-containing heterocyclic moieties. In the forthcoming decade, a much greater share of new nitrogen-based pharmaceuticals is anticipated. Many new nitrogen-based heterocycles have been designed. The number of novel N-heterocyclic moieties with significant physiological properties and promising applications in medicinal chemistry is ever-growing. In this review, we consolidate the recent advances on novel nitrogen-containing heterocycles and their distinct biological activities, reported over the past one year (2019 to early 2020). This review highlights the trends in the use of nitrogen-based moieties in drug design and the development of different potent and competent candidates against various diseases.
Keywords: biological activities; current trends; nitrogen-based heterocycles; structure-activity relationship.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

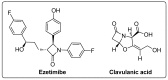


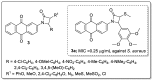
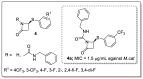

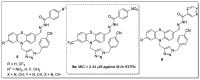
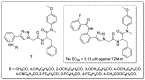


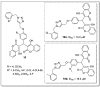

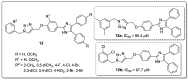
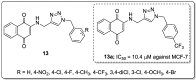




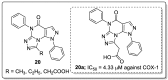

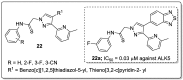

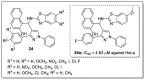

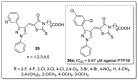

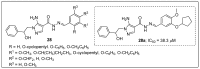
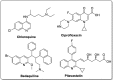
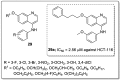

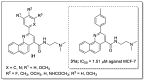
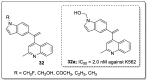
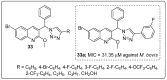
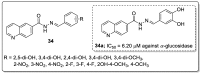
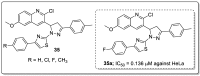


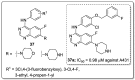
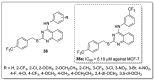
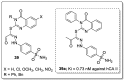


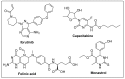

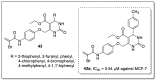
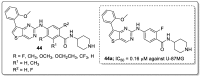
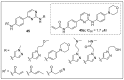
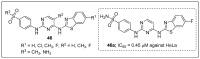
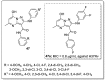
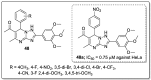
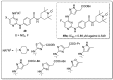
References
-
- Kerru N., Bhaskaruni S.V.H.S., Gummidi L., Maddila S.N., Maddila S., Jonnalagadda S.B. Recent advances in heterogeneous catalysts for the synthesis of imidazole derivatives. Synth. Commun. 2019;49:2437–2459. doi: 10.1080/00397911.2019.1639755. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

