Association of SNP-SNP Interactions of Surfactant Protein Genes with Pediatric Acute Respiratory Failure
- PMID: 32326132
- PMCID: PMC7231046
- DOI: 10.3390/jcm9041183
Association of SNP-SNP Interactions of Surfactant Protein Genes with Pediatric Acute Respiratory Failure
Abstract
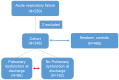
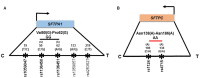
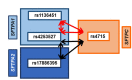
The hallmarks of pediatric acute respiratory failure (ARF) are dysregulated inflammation and surfactant dysfunction. The objective is to study association of surfactant protein (SP) genes' single nucleotide polymorphisms (SNPs) with ARF and its morbidity: pulmonary dysfunction at discharge (PDAD), employing a single-, two-, and three-SNP interaction model. We enrolled 468 newborn controls and 248 children aged ≤ 24 months with ARF; 86 developed PDAD. Using quantitative genetic principles, we tested the association of SP genes SNPs with ARF and PDAD. We observed a dominant effect of rs4715 of the SFTPC on ARF risk. In a three-SNP model, we found (a) 34 significant interactions among SNPs of SFTPA1, SFTPA2, and SFTPC associated with ARF (p = 0.000000002-0.05); 15 and 19 of those interactions were associated with increased and decreased risk for ARF, respectively; (b) intergenic SNP-SNP interactions of both hydrophobic and hydrophilic SP genes associated with PDAD (p = 0.00002-0.03). The majority of intra- and intergenic interactions associated with ARF involve the SFTPA2 SNPs, whereas most of the intra- and intergenic interactions associated with PDAD are of SFTPA1 SNPs. We also observed a dominant effect of haplotypes GG of SFTPA1 associated with increased and AA of SFTPC associated with decreased ARF risk (p = 0.02). To the best of our knowledge, this is the first study showing an association of complex interactions of SP genes with ARF and PDAD. Our data indicate that SP genes polymorphisms may contribute to ARF pathogenesis and subsequent PDAD and/or may serve as markers for disease susceptibility in healthy children.
Keywords: Pediatric acute respiratory failure; SFTPB; SFTPC; SFTPD; SNP–SNP interaction; Surfactant protein gene A1 (SFTPA1), SFTPA2.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hammer J., Eber E. Paediatric Pulmonary Function Testing. Vol. 33. Karger Publishers; Basel, Switzerland: 2005. The peculiarities of infant respiratory physiology; pp. 2–7.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

