New Therapeutic Strategies for Osteoarthritis by Targeting Sialic Acid Receptors
- PMID: 32326143
- PMCID: PMC7226619
- DOI: 10.3390/biom10040637
New Therapeutic Strategies for Osteoarthritis by Targeting Sialic Acid Receptors
Abstract
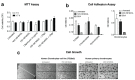
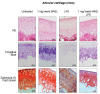
Osteoarthritis (OA) is the most common degenerative joint disease characterized by articular cartilage degradation and joint degeneration. The articular cartilage is mainly formed by chondrocytes and a collagen-proteoglycan extracellular matrix that contains high levels of glycosylated proteins. It was reported that the shift from glycoproteins containing α-2,6-linked sialic acids to those that contain α-2,3 was associated with the onset of common types of arthritis. However, the pathophysiology of α-2,3-sialylation in cartilage has not been yet elucidated. We show that cartilage from osteoarthritic patients expresses high levels of the α-2,3-sialylated transmembrane mucin receptor, known as podoplanin (PDPN). Additionally, the Maackia amurensis seed lectin (MASL), that can be utilized to target PDPN, attenuates the inflammatory response mediated by NF-kB activation in primary chondrocytes and protects human cartilage breakdown ex vivo and in an animal model of arthritis. These findings reveal that specific lectins targeting α-2,3-sialylated receptors on chondrocytes might effectively inhibit cartilage breakdown. We also present a computational 3D molecular model for this interaction. These findings provide mechanistic information on how a specific lectin could be used as a novel therapy to treat degenerative joint diseases such as osteoarthritis.
Keywords: MASL; arthritis; articular chondrocyte; cartilage; glycoproteins; molecular modelling; osteoarthritis; podoplanin; sialylation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Toegel S., Pabst M., Wu S.Q., Grass J., Goldring M.B., Chiari C., Kolb A., Altmann F., Viernstein H., Unger F.M. Phenotype-related differential alpha-2,6- or alpha-2,3-sialylation of glycoprotein N-glycans in human chondrocytes. Osteoarthr. Cartil. 2010;18:240–248. doi: 10.1016/j.joca.2009.09.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FER-2013/Sociedad Española de Reumatología/International
- FEIOMM-2016/Spanish Society for Research on Bone and Mineral Metabolism/International
- PI13/00591/Instituto de Salud Carlos III/International
- PI16/00035/Instituto de Salud Carlos III/International
- CTQ2017-88353-R/Ministerio de Economía, Industria y Competitividad, Gobierno de España/International

