Differential Small RNA Responses against Co-Infecting Insect-Specific Viruses in Aedes albopictus Mosquitoes
- PMID: 32326240
- PMCID: PMC7232154
- DOI: 10.3390/v12040468
Differential Small RNA Responses against Co-Infecting Insect-Specific Viruses in Aedes albopictus Mosquitoes
Abstract
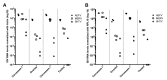
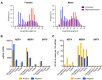
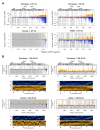
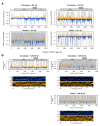
The mosquito antiviral response has mainly been studied in the context of arthropod-borne virus (arbovirus) infection in female mosquitoes. However, in nature, both female and male mosquitoes are frequently infected with insect-specific viruses (ISVs). ISVs are capable of infecting the reproductive organs of both sexes and are primarily maintained by vertical transmission. Since the RNA interference (RNAi)-mediated antiviral response plays an important antiviral role in mosquitoes, ISVs constitute a relevant model to study sex-dependent antiviral responses. Using a naturally generated viral stock containing three distinct ISVs, Aedes flavivirus (AEFV), Menghai rhabdovirus (MERV), and Shinobi tetra virus (SHTV), we infected adult Aedes albopictus females and males and generated small RNA libraries from ovaries, testes, and the remainder of the body. Overall, both female and male mosquitoes showed unique small RNA profiles to each co-infecting ISV regardless of the sex or tissue tested. While all three ISVs generated virus-derived siRNAs, only MERV generated virus-derived piRNAs. We also studied the expression of PIWI genes in reproductive tissues and carcasses. In contrast to Piwi5-9, Piwi1-4 were abundantly expressed in ovaries and testes, suggesting that Piwi5-9 are involved in exogenous viral piRNA production. Together, our results show that ISV-infected Aedes albopictus produce viral small RNAs in a virus-specific manner and that male mosquitoes mount a similar small RNA-mediated antiviral response to that of females.
Keywords: Aedes albopictus; PIWI-interacting RNA; co-infection; insect-specific viruses; reproductive tissues; sex difference; small interfering RNA.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Fauver J.R., Grubaugh N.D., Krajacich B.J., Weger-Lucarelli J., Lakin S.M., Fakoli L.S., Bolay F.K., Diclaro J.W., Dabiré K.R., Foy B., et al. West African Anopheles gambiae mosquitoes harbor a taxonomically diverse virome including new insect-specific flaviviruses, mononegaviruses, and totiviruses. Virology. 2016;498:288–299. doi: 10.1016/j.virol.2016.07.031. - DOI - PubMed
-
- Shi C., Beller L., Deboutte W., Yinda K.C., Delang L., Vega-Rúa A., Failloux A.-B., Matthijnssens J. Stable distinct core eukaryotic viromes in different mosquito species from Guadeloupe, using single mosquito viral metagenomics. Microbiome. 2019;7:121. doi: 10.1186/s40168-019-0734-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

