PDE2A Is Indispensable for Mouse Liver Development and Hematopoiesis
- PMID: 32326334
- PMCID: PMC7215450
- DOI: 10.3390/ijms21082902
PDE2A Is Indispensable for Mouse Liver Development and Hematopoiesis
Abstract
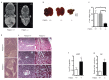
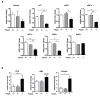
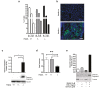
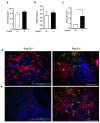
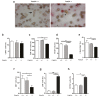
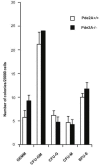
Phosphodiesterase 2A (PDE2A) is a cAMP-cGMP hydrolyzing enzyme essential for mouse development and the PDE2A knockout model (PDE2A-/-) is embryonic lethal. Notably, livers of PDE2A-/- embryos at embryonic day 14.5 (E14.5) have extremely reduced size. Morphological, cellular and molecular analyses revealed loss of integrity in the PDE2A-/- liver niche that compromises the hematopoietic function and maturation. Hematopoietic cells isolated from PDE2A-/- livers are instead able to differentiate in in vitro assays, suggesting the absence of blood cell-autonomous defects. Apoptosis was revealed in hepatoblasts and at the endothelial and stromal compartments in livers of PDE2A-/- embryos. The increase of the intracellular cAMP level and of the inducible cAMP early repressor (ICER) in liver of PDE2A-/- embryos might explain the impairment of liver development by downregulating the expression of the anti-apoptotic gene Bcl2. In summary, we propose PDE2A as an essential gene for integrity maintenance of liver niche and the accomplishment of hematopoiesis.
Keywords: ICER; apoptosis; cAMP; fetal liver; hematopoiesis; phosphodiesterase 2A.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

