The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem
- PMID: 32326444
- PMCID: PMC7226971
- DOI: 10.3390/cells9040976
The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem
Abstract
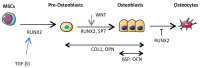
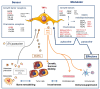
Osteosarcomas are the most frequent primary bone sarcomas, affecting mainly children, adolescents, and young adults, and with a second peak of incidence in elderly individuals. The current therapeutic management, a combined regimen of poly-chemotherapy and surgery, still remains largely insufficient, as patient survival has not improved in recent decades. Osteosarcomas are very heterogeneous tumors, both at the intra- and inter-tumor level, with no identified driver mutation. Consequently, efforts to improve treatments using targeted therapies have faced this lack of specific osteosarcoma targets. Nevertheless, these tumors are inextricably linked to their local microenvironment, composed of bone, stromal, vascular and immune cells and the osteosarcoma microenvironment is now considered to be essential and supportive for growth and dissemination. This review describes the different actors of the osteosarcoma microenvironment and gives an overview of the past, current, and future strategies of therapy targeting this complex ecosystem, with a focus on the role of extracellular vesicles and on the emergence of multi-kinase inhibitors.
Keywords: bone; extracellular vesicles; microenvironment; multi-kinase inhibitors; osteosarcoma; stromal cells; targeted therapies; vascular cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gaebler M., Silvestri A., Haybaeck J., Reichardt P., Lowery C.D., Stancato L.F., Zybarth G., Regenbrecht C.R.A. Three-Dimensional Patient-Derived In Vitro Sarcoma Models: Promising Tools for Improving Clinical Tumor Management. Front. Oncol. 2017;7:203. doi: 10.3389/fonc.2017.00203. - DOI - PMC - PubMed
-
- Gaspar N., Occean B.V., Pacquement H., Bompas E., Bouvier C., Brisse H.J., Castex M.P., Cheurfa N., Corradini N., Delaye J., et al. Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. Eur. J. Cancer. 2018;88:57–66. doi: 10.1016/j.ejca.2017.09.036. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

