Imaging Transcranial Direct Current Stimulation (tDCS) with Positron Emission Tomography (PET)
- PMID: 32326515
- PMCID: PMC7226010
- DOI: 10.3390/brainsci10040236
Imaging Transcranial Direct Current Stimulation (tDCS) with Positron Emission Tomography (PET)
Abstract
Transcranial direct current stimulation (tDCS) is a form of non-invasive neuromodulation that is increasingly being utilized to examine and modify several cognitive and motor functions. Although tDCS holds great potential, it is difficult to determine optimal treatment procedures to accommodate configurations, the complex shapes, and dramatic conductivity differences among various tissues. Furthermore, recent demonstrations showed that up to 75% of the tDCS current applied to rodents and human cadavers was shunted by the scalp, subcutaneous tissue, and muscle, bringing the effects of tDCS on the cortex into question. Consequently, it is essential to combine tDCS with human neuroimaging to complement animal and cadaver studies and clarify if and how tDCS can affect neural function. One viable approach is positron emission tomography (PET) imaging. PET has unique potential for examining the effects of tDCS within the central nervous system in vivo, including cerebral metabolism, neuroreceptor occupancy, and neurotransmitter activity/binding. The focus of this review is the emerging role of PET and potential PET radiotracers for studying tDCS-induced functional changes in the human brain.
Keywords: cortical excitability; positron emission tomography; radiotracers; tDCS.
Conflict of interest statement
The authors declare no conflict of interest.
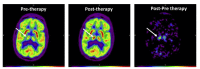
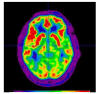
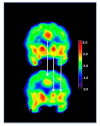
Figures

References
-
- Buch E.R., Santamecchi E., Antal A., Born J., Celnik P.A., Classen J., Gerloffi C., Hallett M., Hummeli F.C., Nitschek M.A., et al. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clin. Neurophysiol. 2017;128:589–603. doi: 10.1016/j.clinph.2017.01.004. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

