The integrated stress response: From mechanism to disease
- PMID: 32327570
- PMCID: PMC8997189
- DOI: 10.1126/science.aat5314
The integrated stress response: From mechanism to disease
Abstract
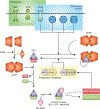
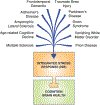
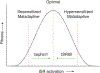
Protein quality control is essential for the proper function of cells and the organisms that they make up. The resulting loss of proteostasis, the processes by which the health of the cell's proteins is monitored and maintained at homeostasis, is associated with a wide range of age-related human diseases. Here, we highlight how the integrated stress response (ISR), a central signaling network that responds to proteostasis defects by tuning protein synthesis rates, impedes the formation of long-term memory. In addition, we address how dysregulated ISR signaling contributes to the pathogenesis of complex diseases, including cognitive disorders, neurodegeneration, cancer, diabetes, and metabolic disorders. The development of tools through which the ISR can be modulated promises to uncover new avenues to diminish pathologies resulting from it for clinical benefit.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

