Robust autoactivation for apoptosis by BAK but not BAX highlights BAK as an important therapeutic target
- PMID: 32327636
- PMCID: PMC7181796
- DOI: 10.1038/s41419-020-2463-7
Robust autoactivation for apoptosis by BAK but not BAX highlights BAK as an important therapeutic target
Abstract
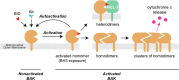
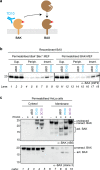
BAK and BAX, which drive commitment to apoptosis, are activated principally by certain BH3-only proteins that bind them and trigger major rearrangements. One crucial conformation change is exposure of their BH3 domain which allows BAK or BAX to form homodimers, and potentially to autoactivate other BAK and BAX molecules to ensure robust pore formation and cell death. Here, we test whether full-length BAK or mitochondrial BAX that are specifically activated by antibodies can then activate other BAK or BAX molecules. We found that antibody-activated BAK efficiently activated BAK as well as mitochondrial or cytosolic BAX, but antibody-activated BAX unexpectedly proved a poor activator. Notably, autoactivation by BAK involved transient interactions, as BAK and BAX molecules it activated could dissociate and homodimerize. The results suggest that BAK-driven autoactivation may play a substantial role in apoptosis, including recruitment of BAX to the mitochondria. Hence, directly targeting BAK rather than BAX may prove particularly effective in inhibiting unwanted apoptosis, or alternatively, inducing apoptosis in cancer cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Bock, F. J. & Tait, S. W. G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 10.1038/s41580-019-0173-8 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

