High temperatures alter cross-over distribution and induce male meiotic restitution in Arabidopsis thaliana
- PMID: 32327690
- PMCID: PMC7181631
- DOI: 10.1038/s42003-020-0897-1
High temperatures alter cross-over distribution and induce male meiotic restitution in Arabidopsis thaliana
Abstract
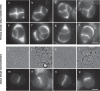
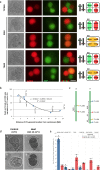
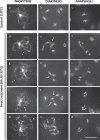
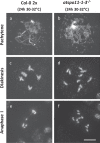
Plant fertility is highly sensitive to elevated temperature. Here, we report that hot spells induce the formation of dyads and triads by disrupting the biogenesis or stability of the radial microtubule arrays (RMAs) at telophase II. Heat-induced meiotic restitution in Arabidopsis is predominantly SDR-type (Second Division Restitution) indicating specific interference with RMAs formed between separated sister chromatids. In addition, elevated temperatures caused distinct deviations in cross-over formation in male meiosis. Synapsis at pachytene was impaired and the obligate cross-over per chromosome was discarded, resulting in partial univalency in meiosis I (MI). At diakinesis, interconnections between non-homologous chromosomes tied separate bivalents together, suggesting heat induces ectopic events of non-homologous recombination. Summarized, heat interferes with male meiotic cross-over designation and cell wall formation, providing a mechanistic basis for plant karyotype change and genome evolution under high temperature conditions.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Hatfield JerryL, John HPrueger. “Temperature extremes: effect on plant growth and development.”. Weather Clim. extremes. 2015;10:4–10. doi: 10.1016/j.wace.2015.08.001. - DOI
-
- Negri V, Lemmi G. Effect of selection and temperature stress on the production of 2n gametes in Lotus tenuis. Plant Breed. 1998;117:345–349. doi: 10.1111/j.1439-0523.1998.tb01952.x. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

