PRMT5 Is Required for T Cell Survival and Proliferation by Maintaining Cytokine Signaling
- PMID: 32328070
- PMCID: PMC7160866
- DOI: 10.3389/fimmu.2020.00621
PRMT5 Is Required for T Cell Survival and Proliferation by Maintaining Cytokine Signaling
Abstract
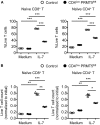
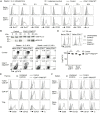
Arginine methylation is a post-translational modification that regulates many biological processes. However, the role of arginine methylation in immune cells is not well studied. Here we report an essential role of protein arginine methyltransferase 5 (PRMT5) in T cell homeostasis and activation-induced expansion. Using T cell-specific PRMT5 conditional knockout mice, we found that PRMT5 is required for natural killer T (NKT) cell but not for conventional or regulatory T (Treg) cell development after the double positive (DP) stage in the thymus. In contrast, PRMT5 was required for optimal peripheral T cell maintenance, for the transition of naïve T cells to effector/memory phenotype, and for early T cell development before the DP stage in a cell-intrinsic manner. Accordingly, PRMT5-deleted T cells showed impaired IL-7-mediated survival and TCR-induced proliferation in vitro. The latter was more pronounced and attributed to reduced responsiveness to IL-2. Acute deletion of PRMT5 revealed that not only naïve but also effector/memory T cells were impaired in TCR-induced proliferation in a development-independent manner. Reduced expression of common γ chain (γc), a shared receptor component for several cytokines including IL-7 and IL-2, on PRMT5-deleted T cells may be in part responsible for the defect. We further showed that PRMT5 was partially required for homeostatic T cell survival but absolutely required for lymphopenic T cell expansion in vivo. Thus, we propose that PRMT5 is required for T cell survival and proliferation by maintaining cytokine signaling, especially during proliferation. The inhibition of PRMT5 may provide a novel strategy for the treatment of diseases where uncontrolled T cell activation has a role, such as autoimmunity.
Keywords: PRMT5; T cell development; T cell proliferation; T cell survival; arginine methylation; cytokine signaling.
Copyright © 2020 Tanaka, Nagai, Okumura, Greene and Kambayashi.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

