Supramolecular Assembly of Thiophene-Based Oligomers into Nanostructured Fluorescent Conductive and Chiral Microfibers
- PMID: 32328405
- PMCID: PMC7175019
- DOI: 10.1002/open.201900347
Supramolecular Assembly of Thiophene-Based Oligomers into Nanostructured Fluorescent Conductive and Chiral Microfibers
Abstract
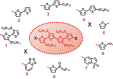
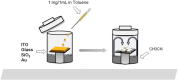
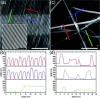
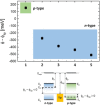
The implementation of nano/microelectronic devices requires efficient strategies for the realization of supramolecular structures with desired function and supported on appropriate substrates. This article illustrates a strategy based on the synthesis of thiophene oligomers having the same "sulfur-overrich" quaterthiophene inner core (non bonding interactional algorithm) and different terminal groups. Nano/microfibers are formed on surfaces having a morphology independent of the nature of the deposition substrate and displaying a wide tuning of properties that make the fibers optoelectronically suitable for application in devices.
Keywords: charge conduction; chirality; nano/microfibers; self-assembly; sulfur-overrich thiophenes.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- None
-
- Xu K., Xie S., Instrum. Sci. Technol. 2019. https://doi.org/10.1080/10739149.2019.1660182;
-
- Chochos C. L., Spanos M., Katsouras A., Tatsi E., Drakopoulou S., Gregoriou V. G., Avgeropoulos A., Prog. Polym. Sci. 2019, 91, 51–79;
-
- Ouchi H., Lin X., Yagai S., Chem. Lett. 2019, 48, 1009–1018;
-
- V. Vohra, T. Anzai. J. Nanomater 2017, vol. 2017, Article ID 3624750, 18 pages. https://doi.org/10.1155/2017/3624750;
Publication types
LinkOut - more resources
Full Text Sources

