5-Aminouracil and other inhibitors of DNA replication induce biphasic interphase-mitotic cells in apical root meristems of Allium cepa
- PMID: 32328702
- PMCID: PMC7359111
- DOI: 10.1007/s00299-020-02545-9
5-Aminouracil and other inhibitors of DNA replication induce biphasic interphase-mitotic cells in apical root meristems of Allium cepa
Abstract
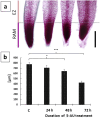
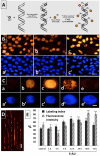
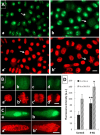
Induction of biphasic interphase-mitotic cells and PCC is connected with an increased level of metabolism in root meristem cells of Allium cepa. Previous experiments using primary roots of Allium cepa exposed to low concentrations of hydroxyurea have shown that long-term DNA replication stress (DRS) disrupts essential links of the S-M checkpoint mechanism, leading meristem cells either to premature chromosome condensation (PCC) or to a specific form of chromatin condensation, establishing biphasic organization of cell nuclei with both interphase and mitotic domains (IM cells). The present study supplements and extends these observations by describing general conditions under which both abnormal types of M-phase cells may occur. The analysis of root apical meristem (RAM) cell proliferation after prolonged mild DRS indicates that a broad spectrum of inhibitors is capable of generating PCC and IM organization of cell nuclei. These included: 5-aminouracil (5-AU, a thymine antagonist), characterized by the highest efficiency in creating cells with the IM phenotype, aphidicolin (APH), an inhibitor of DNA polymerase α, 5-fluorodeoxyuridine (FUdR), an inhibitor of thymidylate synthetase, methotrexate (MTX), a folic acid analog that inhibits purine and pyrimidine synthesis, and cytosine arabinoside (Ara-C), which inhibits DNA replication by forming cleavage complexes with topoisomerase I. As evidenced using fluorescence-based click chemistry assays, continuous treatment of onion RAM cells with 5-AU is associated with an accelerated dynamics of the DNA replication machinery and significantly enhanced levels of transcription and translation. Furthermore, DRS conditions bring about an intensified production of hydrogen peroxide (H2O2), depletion of reduced glutathione (GSH), and some increase in DNA fragmentation, associated with only a slight increase in apoptosis-like programmed cell death events.
Keywords: 5-AU; Allium cepa; IM cells; Metabolic activity; ROS; Replication stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

