Generation of Recombinant Mammalian Selenoproteins through Genetic Code Expansion with Photocaged Selenocysteine
- PMID: 32330002
- PMCID: PMC7372505
- DOI: 10.1021/acschembio.0c00147
Generation of Recombinant Mammalian Selenoproteins through Genetic Code Expansion with Photocaged Selenocysteine
Abstract
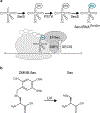
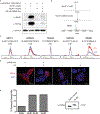
Selenoproteins contain the amino acid selenocysteine (Sec) and are found in all domains of life. The functions of many selenoproteins are poorly understood, partly due to difficulties in producing recombinant selenoproteins for cell-biological evaluation. Endogenous mammalian selenoproteins are produced through a noncanonical translation mechanism requiring suppression of the UGA stop codon and a Sec insertion sequence (SECIS) element in the 3' untranslated region of the mRNA. Here, recombinant selenoproteins are generated in mammalian cells through genetic code expansion, circumventing the requirement for the SECIS element and selenium availability. An engineered orthogonal E. coli leucyl-tRNA synthetase/tRNA pair is used to incorporate a photocaged Sec (DMNB-Sec) at the UAG amber stop codon. DMNB-Sec is successfully incorporated into GFP and uncaged by irradiation of living cells. Furthermore, DMNB-Sec is used to generate the native selenoprotein methionine-R-sulfoxide reductase B1 (MsrB1). Importantly, MsrB1 is shown to be catalytically active after uncaging, constituting the first use of genetic code expansion to generate a functional selenoprotein in mammalian systems. The ability to site-specifically introduce Sec directly in mammalian cells, and temporally modulate selenoprotein activity, will aid in the characterization of mammalian selenoprotein function.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Kryukov GV et al. Characterization of mammalian selenoproteomes. Science 300, 1439–1443 (2003). - PubMed
-
- Reich HJ & Hondal RJ Why Nature Chose Selenium. ACS Chem Biol 11, 821–841 (2016). - PubMed
-
- Ingold I et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 172, 409–422 e421 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

