A phase 1/2 study of the oral FLT3 inhibitor pexidartinib in relapsed/refractory FLT3-ITD-mutant acute myeloid leukemia
- PMID: 32330242
- PMCID: PMC7189289
- DOI: 10.1182/bloodadvances.2020001449
A phase 1/2 study of the oral FLT3 inhibitor pexidartinib in relapsed/refractory FLT3-ITD-mutant acute myeloid leukemia
Abstract
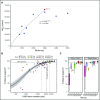
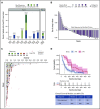
FMS-like tyrosine kinase 3 (FLT3) tyrosine kinase inhibitors (TKIs) have activity in acute myeloid leukemia (AML) patients with FLT3 internal tandem duplication (ITD) mutations, but efficacy is limited by resistance-conferring kinase domain mutations. This phase 1/2 study evaluated the safety, tolerability, and efficacy of the oral FLT3 inhibitor PLX3397 (pexidartinib), which has activity against the FLT3 TKI-resistant F691L gatekeeper mutation in relapsed/refractory FLT3-ITD-mutant AML. Ninety patients were treated: 34 in dose escalation (part 1) and 56 in dose expansion (part 2). Doses of 800 to 5000 mg per day in divided doses were tested. No maximally tolerated dose was reached. Plasma inhibitory assay demonstrated that patients dosed with ≥3000 mg had sufficient levels of active drug in their trough plasma samples to achieve 95% inhibition of FLT3 phosphorylation in an FLT3-ITD AML cell line. Based on a plateau in drug exposure, the 3000-mg dose was chosen as the recommended phase 2 dose. The most frequently reported treatment-emergent adverse events were diarrhea (50%), fatigue (47%), and nausea (46%). Based on modified response criteria, the overall response rate to pexidartinib among all patients was 21%. Twenty-three percent of patients treated at ≥2000 mg responded. The overall composite complete response rate for the study was 11%. Six patients were successfully bridged to transplantation. Median overall survival (OS) of patients treated in dose expansion was 112 days (90% confidence interval [CI], 77-150 days), and median OS of responders with complete remission with or without recovery of blood counts was 265 days (90% CI, 170-422 days). This trial was registered at www.clinicaltrials.gov as #NCT01349049.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.C.S. has received research funding from Astellas Pharma, Revolution Medicines, Plexxikon, and FujiFilm and honoraria from Amgen, Astellas Pharma, and Daiichi Sankyo. M.J.L. has received honoraria and research funding from Astellas Pharma, FujiFilm, and Novartis and honoraria from Menarini, Agios, Amgen, and Daiichi Sankyo. A.E.P. has received honoraria from Astellas Pharma, Arog, AbbVie, Actinium Pharmaceuticals, Agios, Jazz, NewLink Genetics, Takeda, and Novartis and research funding from BioMed Valley Discoveries, Daiichi Sankyo, Bayer, and FujiFilm. J.M.P. has received payments for consultancy from Actinium Pharmaceuticals, Gilead, AstraZeneca, and Pharmacyclics. G.J.R. has consulted for and served on advisory committees for AbbVie, Actinium, Agios, Amphivena, Argenx, Array Biopharma, Astex, Astellas, AstraZeneca, Bayer, Celgene, Celltrion, Daiichi Sankyo, Eisai, Epizyme, Helsinn, Janssen, Jasper Therapeutics, Jazz, MEI Pharma (independent data monitoring committee chair), Novartis, Orsenix, Otsuka, Pfizer, Roche/Genentech, Sandoz, Takeda (independent review committee chair), and Trovagene and received research support from Cellectis. E.S.W. has served as an advisor for AbbVie, Kite, Jazz, Astellas, Daiichi Sankyo, Amgen, and Agios and served on speaker’s bureaus and as an advisor for Celyad, Pfizer, and Stemline. M.H.L., H.H.H., P.L.S., C.Z., B.L.W., and G.B. are or were employees of Plexxikon. The remaining authors declare no competing financial interests.
Figures

References
-
- Papaemmanuil E, Döhner H, Campbell PJ. Genomic classification in acute myeloid leukemia. N Engl J Med. 2016;375(9):900-901. - PubMed
-
- Fröhling S, Schlenk RF, Breitruck J, et al. ; AML Study Group Ulm. Acute myeloid leukemia . Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372-4380. - PubMed
-
- Kottaridis PD, Gale RE, Frew ME, et al. . The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752-1759. - PubMed
-
- Moreno I, Martín G, Bolufer P, et al. . Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88(1):19-24. - PubMed

