Clinical value of event-free survival in acute myeloid leukemia
- PMID: 32330243
- PMCID: PMC7189295
- DOI: 10.1182/bloodadvances.2019001150
Clinical value of event-free survival in acute myeloid leukemia
Abstract
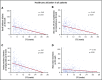
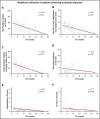
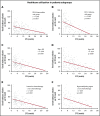
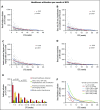
The value of event-free survival (EFS) as an end point in acute myeloid leukemia (AML) trials has been questioned. We hypothesized that rather than a surrogate for overall survival (OS), improvement in EFS may decrease the use of health care. In this retrospective study, we identified 400 patients with AML who were treated on first-line therapy trials and had OS between 2 and 36 months. We captured health care use from diagnosis until death or until the patient was censored at stem cell transplantation (SCT). We used correlation and regression analysis to determine the relation between health care use and EFS. Among patients with newly diagnosed AML, 35% had adverse-risk AML, 48% received intensive chemotherapy, and 28% received hypomethylating agents. The median EFS censored at SCT was 9.7 months. Longer EFS led to a significant decline in health care use regardless of OS. This held true for all observations, including overall health care use (r = -0.45), sum of clinic visits, emergency room visits, hospitalizations, consultations (r = -0.44), sum of invasive procedures, laboratory and imaging studies (r = -0.51), and blood product transfusions (r = -0.19). These correlations were stronger for patients who achieved a complete remission and held true across age, treatment, and disease risk subgroups. In patients with newly diagnosed AML, improvement in EFS correlates with a decrease in all health care use irrespective of OS duration.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.M. received research funding from Celgene. H.M.K. received honoraria and research funding from Pfizer, ARIAD, Bristol-Myers Squibb, and Amgen, honoraria from Orsenix, Immunogen, Actinium, and AbbVie, and research funding from Novartis and Astex. M.Y.K. received research funding from Stemline Therapeutics. S.V. received research funding from Genentech, Lilly Oncology, NS Pharma, CTI BioPharma, Incyte, Seattle Genetics, Blueprint Medicines, Celgene, Pfizer, Roche, AstraZeneca, Bristol-Myers Squibb, Promedior, Galena BioPharma, and Gilead. M.A. received research funding from AstraZeneca. H.M.K. received honoraria and research funding from Pfizer, Bristol-Myers Squibb, ARIAD, and Amgen, honoraria from Orsenix, Immunogen, Actinium, and AbbVie, and research funding from Novartis and Astex. T.M.K. has served as a consultant for and received research funding from Amgen, Jazz Pharmaceuticals, and Pfizer, served as a consultant for Takeda, Novartis, and AbbVie, and received research funding from Celgene and Bristol-Myers Squibb. N.G.D. served as a consultant for and received research funding from Incyte, Daiichi-Sankyo, ImmunoGen, Karyopharm, Bristol-Myers Squibb, Pfizer, and Novartis, served as a consultant for Sunesis, Alexion, and Otsuka, and has received research funding from Pfizer, Novartis, Kiromic, and ARIAD. N.P. served as a consultant for and received honoraria from Celgene, and has served as a consultant for and received honoraria and research funding from Stemline. C.D.D. served as a consultant and adviser for AbbVie and Agios, as an adviser for Bayer, Celgene, Medimmune, and Karyopharm, and received research funding from Plexxikon, Novartis, Affymetrix, Samus, Cellectis, SagerStrong Foundation, AbbVie, and Daiichi Sankyo. W.G.W. received research funding from AbbVie and Genentech. F.R. has received honoraria and research funding from and served on the Speakers Bureau for Amgen, has received honoraria from Jazz Pharmaceuticals, Sunesis, and Orsenix, has received research funding from AbbVie, Xencor, Bristol-Myers Squibb, and Seattle Genetics, has received honoraria and research funding from Macrogenix, and has served as a consultant for and received honoraria from Astellas Pharmaceuticals. J.E.C. has received research funding from Amphivena, Arog, Astellas Pharma, Bristol-Myers Squibb, Celgene, Daiichi-Sankyo, Immunogen, Jazz Pharmaceuticals, Merus, Novartis, Pfizer, Sun Pharma, Takeda, Teva, and Rafael, and has served as a consultant for Astellas Pharma, BiolineRx, Biopath Holdings, Bristol-Myers Squibb, Daiichi-Sankyo, Novartis, Pfizer, Takeda, and Jazz Pharmaceuticals (all to the institution). The remaining authors declare no competing financial interests.
Figures

References
-
- Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099-2107. - PubMed
-
- National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Acute Myeloid Leukemia (AML). Available at: https://seer.cancer.gov/statfacts/html/amyl.html Accessed 1 January 2018.
-
- Luskin MR, Lee J-W, Fernandez HF, et al. . Results of the ECOG E1900 trial in younger adults with AML using an event free survival endpoint are concordant with results based on overall survival: Potential for a surrogate endpoint to facilitate rapid approval of therapies in AML [abstract]. Blood. 2014;124(21). Abstract 2599.
-
- Yin J, Uy GL, Laplant MS, et al. . Event-free survival as a surrogate endpoint for overall survival in previously untreated acute myeloid leukemia: An individual patient-level analysis of multiple randomized trials (Alliance A151614) [abstract]. Blood. 2018;132(suppl 1). Abstract 1386.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

