Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial
- PMID: 32330277
- PMCID: PMC12124691
- DOI: 10.1001/jamanetworkopen.2020.8857
Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial
Abstract
Importance: There is no specific antiviral therapy recommended for coronavirus disease 2019 (COVID-19). In vitro studies indicate that the antiviral effect of chloroquine diphosphate (CQ) requires a high concentration of the drug.
Objective: To evaluate the safety and efficacy of 2 CQ dosages in patients with severe COVID-19.
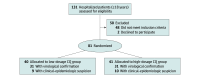
Design, setting, and participants: This parallel, double-masked, randomized, phase IIb clinical trial with 81 adult patients who were hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was conducted from March 23 to April 5, 2020, at a tertiary care facility in Manaus, Brazilian Amazon.
Interventions: Patients were allocated to receive high-dosage CQ (ie, 600 mg CQ twice daily for 10 days) or low-dosage CQ (ie, 450 mg twice daily on day 1 and once daily for 4 days).
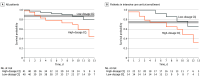
Main outcomes and measures: Primary outcome was reduction in lethality by at least 50% in the high-dosage group compared with the low-dosage group. Data presented here refer primarily to safety and lethality outcomes during treatment on day 13. Secondary end points included participant clinical status, laboratory examinations, and electrocardiogram results. Outcomes will be presented to day 28. Viral respiratory secretion RNA detection was performed on days 0 and 4.
Results: Out of a predefined sample size of 440 patients, 81 were enrolled (41 [50.6%] to high-dosage group and 40 [49.4%] to low-dosage group). Enrolled patients had a mean (SD) age of 51.1 (13.9) years, and most (60 [75.3%]) were men. Older age (mean [SD] age, 54.7 [13.7] years vs 47.4 [13.3] years) and more heart disease (5 of 28 [17.9%] vs 0) were seen in the high-dose group. Viral RNA was detected in 31 of 40 (77.5%) and 31 of 41 (75.6%) patients in the low-dosage and high-dosage groups, respectively. Lethality until day 13 was 39.0% in the high-dosage group (16 of 41) and 15.0% in the low-dosage group (6 of 40). The high-dosage group presented more instance of QTc interval greater than 500 milliseconds (7 of 37 [18.9%]) compared with the low-dosage group (4 of 36 [11.1%]). Respiratory secretion at day 4 was negative in only 6 of 27 patients (22.2%).
Conclusions and relevance: The preliminary findings of this study suggest that the higher CQ dosage should not be recommended for critically ill patients with COVID-19 because of its potential safety hazards, especially when taken concurrently with azithromycin and oseltamivir. These findings cannot be extrapolated to patients with nonsevere COVID-19.
Trial registration: ClinicalTrials.gov Identifier: NCT04323527.
Conflict of interest statement
Figures
Comment in
-
Caution Needed on the Use of Chloroquine and Hydroxychloroquine for Coronavirus Disease 2019.JAMA Netw Open. 2020 Apr 24;3(4):e209035. doi: 10.1001/jamanetworkopen.2020.9035. JAMA Netw Open. 2020. PMID: 32330276 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

