Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: an Italian experience
- PMID: 32330291
- PMCID: PMC7264663
- DOI: 10.1002/jmv.25932
Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: an Italian experience
Abstract
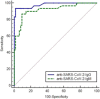
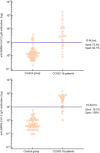
A pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been spreading throughout the world. Though molecular diagnostic tests are the gold standard for COVID-19, serological testing is emerging as a potential surveillance tool, in addition to its complementary role in COVID-19 diagnostics. Indubitably quantitative serological testing provides greater advantages than qualitative tests but today there is still little known about serological diagnostics and what the most appropriate role quantitative tests might play. Sixty-one COVID-19 patients and 64 patients from a control group were tested by iFlash1800 CLIA analyzer for anti-SARS CoV-2 antibodies IgM and IgG. All COVID-19 patients were hospitalized in San Giovanni di Dio Hospital (Florence, Italy) and had a positive oro/nasopharyngeal swab reverse-transcription polymerase chain reaction result. The highest sensitivity with a very good specificity performance was reached at a cutoff value of 10.0 AU/mL for IgM and of 7.1 for IgG antibodies, hence near to the manufacturer's cutoff values of 10 AU/mL for both isotypes. The receiver operating characteristic curves showed area under the curve values of 0.918 and 0.980 for anti-SARS CoV-2 antibodies IgM and IgG, respectively. iFlash1800 CLIA analyzer has shown highly accurate results for the anti-SARS-CoV-2 antibodies profile and can be considered an excellent tool for COVID-19 diagnostics.
Keywords: SARS coronavirus; coronavirus; humoral immunity; immune responses; virus classification.
© 2020 Wiley Periodicals, Inc.
Figures
References
-
- World Health Organization. (2020) . Laboratory testing for coronavirus disease 2019 (COVID‐19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization.
-
- Infantino M, Damiani A, Gobbi FL, et al. Serological assays for SARS‐CoV‐2 infectious disease: benefits, limitations and perspectives. Isr Med Assoc J. 2020;22:203‐210. - PubMed
-
- Okba NMA, Müller MA, Li W. SARS‐CoV‐2 specific antibody responses in COVID‐19 patients. medRxiv. 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous