The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review
- PMID: 32331408
- PMCID: PMC7221839
- DOI: 10.3390/molecules25081941
The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review
Abstract
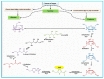
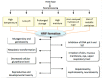
The incidence of adverse reactions in food is very low, however, some food products contain toxins formed naturally due to their handling, processing and storage conditions. 5-(Hydroxymethyl)-2-furfural (HMF) can be formed by hydrogenation of sugar substances in some of manufactured foodstuffs and honey under elevated temperatures and reduced pH conditions following Maillard reactions. In previous studies, it was found that HMF was responsible for harmful (mutagenic, genotoxic, cytotoxic and enzyme inhibitory) effects on human health. HMF occurs in a wide variety of food products like dried fruit, juice, caramel products, coffee, bakery, malt and vinegar. The formation of HMF is not only an indicator of food storage conditions and quality, but HMF could also be used as an indicator of the potential occurrence of contamination during heat-processing of some food products such as coffee, milk, honey and processed fruits. This review focuses on HMF formation and summarizes the adverse effects of HMF on human health.
Keywords: 5-hydroxymethylfurfural; Maillard reaction; heat-borne; toxicant; toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rada-Mendoza M., Sanz M., Olano A., Villamiel M. Formation of hydroxymethylfurfural and furosine during the storage of jams and fruit-based infant foods. Food Chem. 2004;85:605–609. doi: 10.1016/j.foodchem.2003.07.002. - DOI
-
- Chou C.C., Hee S.S.Q. Bioassay-driven analysis of chewing tobacco extracts. Environ. Toxicol. Chem. Int. J. 1994;13:1177–1186. doi: 10.1002/etc.5620130719. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources