Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan
- PMID: 32331982
- PMCID: PMC7194913
- DOI: 10.1016/j.jmii.2020.03.032
Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan
Abstract
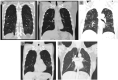
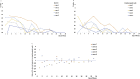
An increase of Ct values was 0.9 per day in 2 cases of COVID-19 treated with lopinavir/ritonavir (LPV/r), an increase was 1.0 per day in 3 cases without LPV/r through illness day 1-10, indicating that LPV/r did not shorten the duration of SARS CoV-2 shedding.
Keywords: COVID-19; Lopinavir/ritonavir (LPV/r); SARS CoV-2; Shedding.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest All authors report no conflicts of interest.
Figures
References
-
- World Health Organization . 2020 Mar 07. Coronavirus disease 2019 (COVID-19) Situation Report–47.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Diagnostic detection of 2019-nCoV by real-time RT-PCR. 2020 Jan 17. https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?s... - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

