Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig
- PMID: 32332765
- PMCID: PMC7265355
- DOI: 10.1038/s41467-020-16048-4
Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig
Abstract
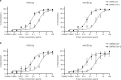
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, at the end of 2019, and there are currently no specific antiviral treatments or vaccines available. SARS-CoV-2 has been shown to use the same cell entry receptor as SARS-CoV, angiotensin-converting enzyme 2 (ACE2). In this report, we generate a recombinant protein by connecting the extracellular domain of human ACE2 to the Fc region of the human immunoglobulin IgG1. A fusion protein containing an ACE2 mutant with low catalytic activity is also used in this study. The fusion proteins are then characterized. Both fusion proteins have a high binding affinity for the receptor-binding domains of SARS-CoV and SARS-CoV-2 and exhibit desirable pharmacological properties in mice. Moreover, the fusion proteins neutralize virus pseudotyped with SARS-CoV or SARS-CoV-2 spike proteins in vitro. As these fusion proteins exhibit cross-reactivity against coronaviruses, they have potential applications in the diagnosis, prophylaxis, and treatment of SARS-CoV-2.
Conflict of interest statement
M.D. is employed by Pharchoice Therapeutics Inc. (Shanghai) and is a shareholder at Pharchoice Therapeutics Inc. (Shanghai). The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- 82041012/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81773261/National Natural Science Foundation of China (National Science Foundation of China)/International
- 31970882/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81903140/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81602690/National Natural Science Foundation of China (National Science Foundation of China)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

