Neuroligin-1 is altered in the hippocampus of Alzheimer's disease patients and mouse models, and modulates the toxicity of amyloid-beta oligomers
- PMID: 32332783
- PMCID: PMC7181681
- DOI: 10.1038/s41598-020-63255-6
Neuroligin-1 is altered in the hippocampus of Alzheimer's disease patients and mouse models, and modulates the toxicity of amyloid-beta oligomers
Abstract
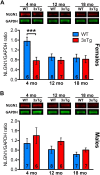
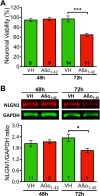
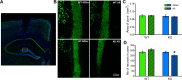
Synapse loss occurs early and correlates with cognitive decline in Alzheimer's disease (AD). Synaptotoxicity is driven, at least in part, by amyloid-beta oligomers (Aβo), but the exact synaptic components targeted by Aβo remain to be identified. We here tested the hypotheses that the post-synaptic protein Neuroligin-1 (NLGN1) is affected early in the process of neurodegeneration in the hippocampus, and specifically by Aβo, and that it can modulate Aβo toxicity. We found that hippocampal NLGN1 was decreased in patients with AD in comparison to patients with mild cognitive impairment and control subjects. Female 3xTg-AD mice also showed a decreased NLGN1 level in the hippocampus at an early age (i.e., 4 months). We observed that chronic hippocampal Aβo injections initially increased the expression of one specific Nlgn1 transcript, which was followed by a clear decrease. Lastly, the absence of NLGN1 decreased neuronal counts in the dentate gyrus, which was not the case in wild-type animals, and worsens impairment in spatial learning following chronic hippocampal Aβo injections. Our findings support that NLGN1 is impacted early during neurodegenerative processes, and that Aβo contributes to this effect. Moreover, our results suggest that the presence of NLGN1 favors the cognitive prognosis during Aβo-driven neurodegeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. Alzheimer’s Disease Neuroimaging Initiative. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog. Neurobiol. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

