Novel Variations in Native Ethiopian Goat breeds PRNP Gene and Their Potential Effect on Prion Protein Stability
- PMID: 32332800
- PMCID: PMC7181617
- DOI: 10.1038/s41598-020-63874-z
Novel Variations in Native Ethiopian Goat breeds PRNP Gene and Their Potential Effect on Prion Protein Stability
Abstract
Scrapie is a lethal neurodegenerative disease of sheep and goats caused by the misfolding of the prion protein. Variants such as M142, D145, S146, H154, Q211, and K222 were experimentally found to increase resistance or extend scrapie incubation period in goats. We aimed to identify polymorphisms in the Afar and Arsi-Bale goat breeds of Ethiopia and computationally assess the effect of variants on prion protein stability. In the present study, four non-synonymous novel polymorphisms G67S, W68R, G69D, and R159H in the first octapeptide repeat and the highly conserved C-terminus globular domain of goat PrP were detected. The resistant genotype, S146, was detected in >50% of the present population. The current study population showed a genetic diversity in Ethiopian goat breeds. In the insilico analysis, the R68 variant was predicted to increase stability while S67, D69, and H159 decrease the stability of prion protein. The new variants in the octapeptide repeat motif were predicted to decrease amyloidogenicity but H159 increased the hotspot sequence amyloidogenic propensity. These novel variants could be the source of conformational flexibility that may trigger the gain or loss of function by prion protein. Further experimental study is required to depict the actual effects of variants on prion protein stability.
Conflict of interest statement
The authors declare no competing interests.
Figures

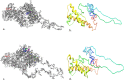
References
-
- Beringue V, Andreoletti O. Classical and atypical TSE in small ruminants. Animal Frontiers. 2014;4:33–43. doi: 10.2527/af.2014-0005. - DOI
-
- Wadsworth, J. D. F. & Collinge, J. In Basic Neurochemistry (Eighth Edition) (eds. Scott T. Brady, George J. Siegel, R. Wayne Albers, & Donald L. Price) 872-885 (Academic Press, 2012).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

