Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD
- PMID: 32333601
- PMCID: PMC7188157
- DOI: 10.1093/ibd/izaa085
Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD
Abstract
Background: Patients with inflammatory bowel disease (IBD) have intestinal inflammation and are treated with immune-modulating medications. In the face of the coronavirus disease-19 pandemic, we do not know whether patients with IBD will be more susceptible to infection or disease. We hypothesized that the viral entry molecules angiotensin I converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) are expressed in the intestine. We further hypothesized that their expression could be affected by inflammation or medication usage.
Methods: We examined the expression of Ace2 and Tmprss2 by quantitative polymerase chain reacion in animal models of IBD. Publicly available data from organoids and mucosal biopsies from patients with IBD were examined for expression of ACE2 and TMPRSS2. We conducted RNA sequencing for CD11b-enriched cells and peripheral and lamina propria T-cells from well-annotated patient samples.
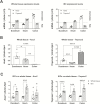
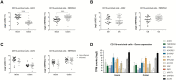
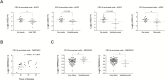
Results: ACE2 and TMPRSS2 were abundantly expressed in the ileum and colon and had high expression in intestinal epithelial cells. In animal models, inflammation led to downregulation of epithelial Ace2. Expression of ACE2 and TMPRSS2 was not increased in samples from patients with compared with those of control patients. In CD11b-enriched cells but not T-cells, the level of expression of ACE2 and TMPRSS2 in the mucosa was comparable to other functional mucosal genes and was not affected by inflammation. Anti-tumor necrosis factor drugs, vedolizumab, ustekinumab, and steroids were linked to significantly lower expression of ACE2 in CD11b-enriched cells.
Conclusions: The viral entry molecules ACE2 and TMPRSS2 are expressed in the ileum and colon. Patients with IBD do not have higher expression during inflammation; medical therapy is associated with lower levels of ACE2. These data provide reassurance for patients with IBD.
Keywords: Crohn disease; coronavirus; ulcerative colitis.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Figures


Comment in
-
The Incidence and Outcomes of COVID-19 in IBD Patients: A Rapid Review and Meta-analysis.Inflamm Bowel Dis. 2020 Sep 18;26(10):e132-e133. doi: 10.1093/ibd/izaa170. Inflamm Bowel Dis. 2020. PMID: 32619003 Free PMC article. No abstract available.
References
-
- WHO. Coronavirus disease 2019 (COVID-19) Situation Report- 74, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

