Depression of Accumbal to Lateral Hypothalamic Synapses Gates Overeating
- PMID: 32333845
- PMCID: PMC7616964
- DOI: 10.1016/j.neuron.2020.03.029
Depression of Accumbal to Lateral Hypothalamic Synapses Gates Overeating
Abstract
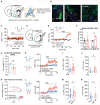
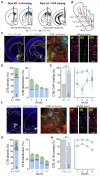
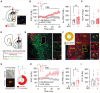
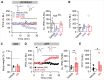
Overeating typically follows periods of energy deficit, but it is also sustained by highly palatable foods, even without metabolic demand. Dopamine D1 receptor-expressing medium spiny neurons (D1-MSNs) of the nucleus accumbens shell (NAcSh) project to the lateral hypothalamus (LH) to authorize feeding when inhibited. Whether plasticity at these synapses can affect food intake is unknown. Here, ex vivo electrophysiology recordings reveal that D1-MSN-to-LH inhibitory transmission is depressed in circumstances in which overeating is promoted. Endocannabinoid signaling is identified as the induction mechanism, since inhibitory plasticity and concomitant overeating were blocked or induced by CB1R antagonism or agonism, respectively. D1-MSN-to-LH projectors were largely non-overlapping with D1-MSNs targeting ventral pallidum or ventral midbrain, providing an anatomical basis for distinct circuit plasticity mechanisms. Our study reveals a critical role for plasticity at D1-MSN-to-LH synapses in adaptive feeding control, which may underlie persistent overeating of unhealthy foods, a major risk factor for developing obesity.
Keywords: VGAT; VGluT2; channelrhodopsin; feeding; hyperphagia; inhibition; lateral hypothalamus; nucleus accumbens; obesity; synaptic plasticity.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests C.L. is a member of the scientific advisory boards of the International Foundation for Research in Paraplegia and the Phenix Foundation. E.C.O. is currently a full-time employee of F. Hoffmann-La Roche AG.
Figures


Comment in
-
Understanding the Munchies.Neuron. 2020 Jul 8;107(1):11-13. doi: 10.1016/j.neuron.2020.06.009. Neuron. 2020. PMID: 32645304
References
-
- Arrigoni E, Chee MJS, Fuller PM. To eat or to sleep: that is a lateral hypothalamic question. Neuropharmacology. 2019;154:34–49. - PubMed
-
- Bocklisch C, Pascoli V, Wong JCY, House DRC, Yvon C, de Roo M, Tan KR, Lüscher C. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science. 2013;341:1521–1525. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

