Baseline immunoreactivity before pregnancy and poly(I:C) dose combine to dictate susceptibility and resilience of offspring to maternal immune activation
- PMID: 32335198
- PMCID: PMC7415552
- DOI: 10.1016/j.bbi.2020.04.061
Baseline immunoreactivity before pregnancy and poly(I:C) dose combine to dictate susceptibility and resilience of offspring to maternal immune activation
Abstract
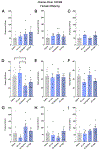
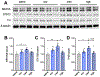
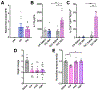
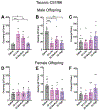
Despite the potential of rodent models of maternal immune activation (MIA) to identify new biomarkers and therapeutic interventions for a range of psychiatric disorders, current approaches using these models ignore two of the most important aspects of this risk factor for human disease: (i) most pregnancies are resilient to maternal viral infection and (ii) susceptible pregnancies can lead to different combinations of phenotypes in offspring. Here, we report two new sources of variability-the baseline immunoreactivity (BIR) of isogenic females prior to pregnancy and differences in immune responses in C57BL/6 dams across vendors-that contribute to resilience and susceptibility to distinct combinations of behavioral and biological outcomes in offspring. Similar to the variable effects of human maternal infection, MIA in mice does not cause disease-related phenotypes in all pregnancies and a combination of poly(I:C) dose and BIR predicts susceptibility and resilience of pregnancies to aberrant repetitive behaviors and alterations in striatal protein levels in offspring. Even more surprising is that the intermediate levels of BIR and poly(I:C) dose are most detrimental to offspring, with higher BIR and poly(I:C) doses conferring resilience to measured phenotypes in offspring. Importantly, we identify the BIR of female mice as a biomarker before pregnancy that predicts which dams will be most at risk as well as biomarkers in the brains of newborn offspring that correlate with changes in repetitive behaviors. Together, our results highlight considerations for optimizing MIA protocols to enhance rigor and reproducibility and reveal new factors that drive susceptibility of some pregnancies and resilience of others to MIA-induced abnormalities in offspring.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
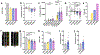


References
-
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET, 2010. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40, 1423–1430. - PubMed
-
- Borta A, Schwarting RK, 2005. Post-trial treatment with the nicotinic agonist metanicotine: Differential effects in Wistar rats with high versus low rearing activity. Pharmacol Biochem Behav 80, 541–548. - PubMed

