Improvement of motor and behavioral activity in Sandhoff mice transplanted with human CD34+ cells transduced with a HexA/HexB expressing lentiviral vector
- PMID: 32335981
- PMCID: PMC12067969
- DOI: 10.1002/jgm.3205
Improvement of motor and behavioral activity in Sandhoff mice transplanted with human CD34+ cells transduced with a HexA/HexB expressing lentiviral vector
Abstract
Background: Tay-Sachs and Sandhoff disease are debilitating genetic diseases that affect the central nervous system leading to neurodegeneration through the accumulation of GM2 gangliosides. There are no cures for these diseases and treatments do not alleviate all symptoms. Hematopoietic stem cell gene therapy offers a promising treatment strategy for delivering wild-type enzymes to affected cells. By genetically modifying hematopoietic stem cells to express wild-type HexA and HexB, systemic delivery of functional enzyme can be achieved.
Methods: Primary human hematopoietic stem/progenitor cells and Tay-Sachs affected cells were used to evaluate the functionality of the vector. An immunodeficient and humanized mouse model of Sandhoff disease was used to evaluate whether the HexA/HexB lentiviral vector transduced cells were able to improve the phenotypes associated with Sandhoff disease. An immunodeficient NOD-RAG1-/-IL2-/- (NRG) mouse model was used to evaluate whether the HexA/HexB vector transduced human CD34+ cells were able to engraft and undergo normal multilineage hematopoiesis.
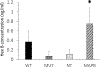
Results: HexA/HexB lentiviral vector transduced cells demonstrated strong expression of HexA and HexB and restored enzyme activity in Tay-Sachs affected cells. Upon transplantation into a humanized Sandhoff disease mouse model, improved motor and behavioral skills were observed. Decreased GM2 gangliosides were observed in the brains of HexA/HexB vector transduced cell transplanted mice. Increased peripheral blood levels of HexB was also observed in transplanted mice. Normal hematopoiesis in the peripheral blood and various lymphoid organs was also observed in transplanted NRG mice.
Conclusions: These results highlight the potential use of stem cell gene therapy as a treatment strategy for Tay-Sachs and Sandhoff disease.
Keywords: gene; hemopoietic; stem cell; therapy; viral vector.
© 2020 John Wiley & Sons, Ltd.
Conflict of interest statement
Figures

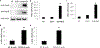
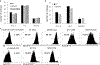


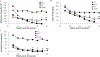
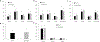
References
-
- Mahuran DJ. Beta-hexosaminidase: biosynthesis and processing of the normal enzyme, and identification of mutations causing Jewish Tay-Sachs disease. Clin Biochem 1995;28:101–106. - PubMed
-
- Mahuran DJ. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim Biophys Acta 1999;1455:105–138. - PubMed
-
- Perlman SL. Late-onset Tay-Sachs disease as a Friedreich ataxia phenocopy. Arch Neurol. 2002;59:1832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

