The Otology Questionnaire Amsterdam: A generic patient-reported outcome measure about the severity and impact of ear complaints. Validation, reliability and responsiveness
- PMID: 32336029
- PMCID: PMC7317857
- DOI: 10.1111/coa.13545
The Otology Questionnaire Amsterdam: A generic patient-reported outcome measure about the severity and impact of ear complaints. Validation, reliability and responsiveness
Abstract
Objective: To examine the construct validity, reliability and responsiveness of the Otology Questionnaire Amsterdam (OQUA).
Design: Multicentre, longitudinal study in 2 separate cohorts of patients visiting an ENT surgeon via an online survey programme.
Setting: Tertiary ENT clinics.
Participants: Cohort 1 consisted of patients at their first visit at an ENT outpatient clinic with an ear complaint. Cohort 2 consisted of patients who underwent surgery, with a 3-month follow-up post-surgery.
Main outcome measures: Construct validity: Hypothesis testing, internal consistency and inter-item correlation. Reliability: Test-retest reliability. The construct approach was used for assessing responsiveness. Hypotheses were formulated based on the association between the OQUA and Glasgow Health Status Inventory (GHSI) or Global Rating Scale (GRS).
Results: Construct validity: The correlation between the individual items in the impact domain ranged from 0.424 to 0.737. Confirmatory factor analysis showed a good fit. As expected, the OQUA impact showed strong relationships with GHSI total and general scale. Reliability: The test-retest reliability coefficient ranged from 0.541 to 0.838. Responsiveness: All hypotheses were conformed. As expected, the change score of the OQUA showed good correlation between OQUA impact and GHSI and moderate correlation between the GRS and OQUA complaints.
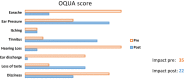
Conclusion: The OQUA has 8 complaint domains (earache, pressure sensation, itching, tinnitus, hearing loss, ear discharge, loss of taste and dizziness) and 1 impact domain. Each domain results in one score of 0-100. The OQUA shows good results for construct validity, (test-retest) reliability and responsiveness, supporting the potential benefit for the patient with an ear complaint visiting the ENT surgeon. The extensive validation furthermore confirms a certified generic otology PROM with an impact and a complaints' part, to be used in different types of otologic interventions and patient groups.
Keywords: audiology; chronic otitis media; mouth; otology; sensorineural hearing loss; taste; tinnitus; vertigo.
© 2020 The Authors. Clinical Otolaryngology published by John Wiley & Sons Ltd.
Conflict of interest statement
None to declare.
Figures


References
-
- Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424‐427. - PubMed
-
- Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143‐148. - PubMed
-
- Philips JS, Haggard M, Yung M. A new health‐related quality of life measure for active chronic otitis media (COMQ‐12): development and initial validation. Otol Neurotol. 2014;35:454‐458. - PubMed
-
- Gates GA, Veral AM. Validation of the ménières disease patient‐oriented symptom‐Severity Index. Arch Otolaryngol Head Neck Surg. 2005;131:863‐867. - PubMed
-
- Bruinewoud EM, Kraak JT, van Leeuwen LM, Kramer SE, Merkus P. The Otology Questionnaire Amsterdam: a generic patient reported outcome measure about the severity and impact of ear complaints. A cross‐sectional study on the development of this questionnaire. Clin Otolaryngol. 2018;43(1):240‐248. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

