Novel approaches and current challenges with targeting the endocannabinoid system
- PMID: 32336154
- PMCID: PMC7502221
- DOI: 10.1080/17460441.2020.1752178
Novel approaches and current challenges with targeting the endocannabinoid system
Abstract
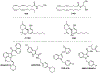
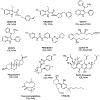
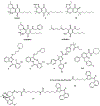
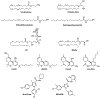
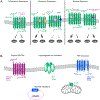
Introduction: The pathophysiological relevance of the endocannabinoid system has been widely demonstrated in a variety of diseases including cancer, neurological disorders, and metabolic issues. Therefore, targeting the receptors and the endogenous machinery involved in this system can provide a successful therapeutic outcome. Ligands targeting the canonical cannabinoid receptors, CB1 and CB2, along with inhibitors of the endocannabinoid enzymes have been thoroughly studied in diverse disease models. In fact, phytocannabinoids such as cannabidiol or Δ9-tetrahydrocannabinol are currently on the market for the management of neuropathic pain due to spasticity in multiple sclerosis or seizures in children epilepsy amongst others.
Areas covered: Challenges in the pharmacology of cannabinoids arise from its pharmacokinetics, off-target effects, and psychoactive effects. In this context, the current review outlines the novel molecular approaches emerging in the field discussing their clinical potential.
Expert opinion: Even if orthosteric CB1 and CB2 ligands are on the forefront in cannabinoid clinical research, emerging strategies such as allosteric or biased modulation of these receptors along with controlled off-targets effects may increase the therapeutic potential of cannabinoids.
Keywords: Cannabinoid; allosteric; bias; mitochondrial; multitarget; off-target; peripheral.
Conflict of interest statement
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Reyes-Resina I, Navarro G, Aguinaga D, et al. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relevance in neurodegenerative diseases. Biochem. Pharmacol 2018;157:169–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
