Study design factors influencing patients' willingness to participate in clinical research: a randomised vignette-based study
- PMID: 32336266
- PMCID: PMC7183682
- DOI: 10.1186/s12874-020-00979-z
Study design factors influencing patients' willingness to participate in clinical research: a randomised vignette-based study
Abstract
Background: High patient participation in clinical research reduces selection bias and ensures the generalizability of study findings. We explored study-related factors that may influence patients' willingness to participate in research.
Methods: We submitted by mail two vignettes that described clinical research studies - a drug trial and a diagnostic study - to patients recently discharged from hospital and assessed their willingness to participate. We used a factorial design to randomly allocate three study attributes per vignette: in the drug trial, presumed superiority of new drug versus equipoise, public versus industry funding, and random versus non-random treatment allocation; in the diagnostic study, common versus rare disease, genetic versus protein analysis, and automatic reporting of results versus reporting on request.
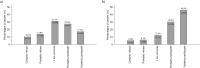
Results: Of 2600 patients contacted, 1140 (44%) participated. Globally, willingness to participate in a drug trial was lower than in a diagnostic study (44.8% vs. 76.2%; P < 0.001). In the drug trial, participation was significantly higher when the new drug was presented as presumably better than the old (vs. equipoise) and when the study was funded by public sources (vs. industry), but was not affected by the allocation method. None of the factors tested in the diagnostic study was associated with participation.
Conclusions: Patients were more likely to participate in a hypothetical observational diagnostic study than in a hypothetical drug trial. Participation in the trial was lower when clinical equipoise was expressed and when the trial was funded by industry. These results suggest that some features of study design can influence participation.
Keywords: Clinical equipoise; Clinical research; Controlled trials; Patient participation; Randomization; Study design.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Lay public's understanding of equipoise and randomisation in randomised controlled trials.Health Technol Assess. 2005 Mar;9(8):1-192, iii-iv. doi: 10.3310/hta9080. Health Technol Assess. 2005. PMID: 15763039
-
Perceptions of equipoise are crucial to trial participation: a qualitative study of men in the ProtecT study.Control Clin Trials. 2003 Jun;24(3):272-82. doi: 10.1016/s0197-2456(03)00020-5. Control Clin Trials. 2003. PMID: 12757993 Clinical Trial.
-
How do study design features and participant characteristics influence willingness to participate in clinical trials? Results from a choice experiment.BMC Med Res Methodol. 2022 Dec 16;22(1):323. doi: 10.1186/s12874-022-01803-6. BMC Med Res Methodol. 2022. PMID: 36526978 Free PMC article.
-
Community equipoise and the architecture of clinical research.Camb Q Healthc Ethics. 1997 Fall;6(4):385-96. doi: 10.1017/s0963180100008136. Camb Q Healthc Ethics. 1997. PMID: 9292216 Review.
-
Influences on clinical trial participation: Enhancing recruitment through a gender lens - A scoping review.Contemp Clin Trials Commun. 2024 Feb 29;38:101283. doi: 10.1016/j.conctc.2024.101283. eCollection 2024 Apr. Contemp Clin Trials Commun. 2024. PMID: 38456181 Free PMC article.
Cited by
-
Applying Nociplastic Pain Criteria in Chronic Musculoskeletal Conditions: A Vignette Study.J Clin Med. 2025 Feb 11;14(4):1179. doi: 10.3390/jcm14041179. J Clin Med. 2025. PMID: 40004711 Free PMC article.
-
Clinical Research: A Review of Study Designs, Hypotheses, Errors, Sampling Types, Ethics, and Informed Consent.Cureus. 2023 Jan 4;15(1):e33374. doi: 10.7759/cureus.33374. eCollection 2023 Jan. Cureus. 2023. PMID: 36751199 Free PMC article. Review.
-
Parental Attitudes toward Consent for Music Intervention Studies in Preterm Infants: A Cross-Sectional Study.Int J Environ Res Public Health. 2021 Jul 28;18(15):7989. doi: 10.3390/ijerph18157989. Int J Environ Res Public Health. 2021. PMID: 34360279 Free PMC article.
-
"I'm tired of seeing my friends die": Barriers and facilitators to participating in clinical trials among rural people who use drugs in the United States.J Rural Health. 2025 Jan;41(1):e70009. doi: 10.1111/jrh.70009. J Rural Health. 2025. PMID: 40045014 Free PMC article.
-
Prospective Preference Assessment of a Pharmacological Clinical Trial to Alter the Progression of Posttraumatic Osteoarthritis After ACL Reconstruction.Orthop J Sports Med. 2025 Mar 11;13(3):23259671241311906. doi: 10.1177/23259671241311906. eCollection 2025 Mar. Orthop J Sports Med. 2025. PMID: 40078595 Free PMC article.
References
-
- Cullati S, Courvoisier DS, Gayet-Ageron A, Haller G, Irion O, Agoritsas T, Rudaz S, Perneger TV. Patient enrollment and logistical problems top the list of difficulties in clinical research: a cross-sectional survey. BMC Med Res Methodol. 2016;16:50. doi: 10.1186/s12874-016-0151-1. - DOI - PMC - PubMed
-
- Kurt A, Kincaid HM, Curtis C, Semler L, Meyers M, Johnson M, Careyva BA, Stello B, Friel TJ, Knouse MC, Smulian JC, Jacoby JL. Factors influencing participation in clinical trials: emergency medicine vs. other specialties. West J Emerg Med. 2017;18:846–855. doi: 10.5811/westjem.2017.5.33827. - DOI - PMC - PubMed
-
- Moocraft SY, Marriott C, Peckitt C, Cunningham D, Chau I, Starling N, Watkins D, Rao S. Patients’ willingness to participate in clinical trials and their views on aspects of cancer research: results of a prospective patient survey. Trials. 2016;17:17–28. doi: 10.1186/s13063-015-1105-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical