Redox toxicology of environmental chemicals causing oxidative stress
- PMID: 32336668
- PMCID: PMC7327986
- DOI: 10.1016/j.redox.2020.101475
Redox toxicology of environmental chemicals causing oxidative stress
Abstract
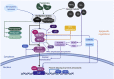
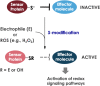
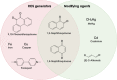
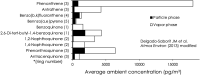
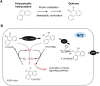
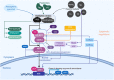
Living organisms are surrounded with heavy metals such as methylmercury, manganese, cobalt, cadmium, arsenic, as well as pesticides such as deltamethrin and paraquat, or atmospheric pollutants such as quinone. Extensive studies have demonstrated a strong link between environmental pollutants and human health. Redox toxicity is proposed as one of the main mechanisms of chemical-induced pathology in humans. Acting as both a sensor of oxidative stress and a positive regulator of antioxidants, the nuclear factor erythroid 2-related factor 2 (NRF2) has attracted recent attention. However, the role NRF2 plays in environmental pollutant-induced toxicity has not been systematically addressed. Here, we characterize NRF2 function in response to various pollutants, such as metals, pesticides and atmospheric quinones. NRF2 related signaling pathways and epigenetic regulations are also reviewed.
Keywords: Air pollutants; Epigenetic modifications; Heavy metals; NRF2; Pesticides; Redox signaling pathways.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Redox Toxicology of Environmental Chemicals Causing Oxidative Stress”.
Figures
References
-
- Ye B.S., Leung A.O.W., Wong M.H. The association of environmental toxicants and autism spectrum disorders in children. Environ. Pollut. 2017;227:234–242. - PubMed
-
- Riancho J. The increasing importance of environmental conditions in amyotrophic lateral sclerosis. Int. J. Biometeorol. 2018;62(8):1361–1374. - PubMed
-
- Cosselman K.E., Navas-Acien A., Kaufman J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 2015;12(11):627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

