EBUS-FNA cytologic-histologic correlation of PD-L1 immunohistochemistry in non-small cell lung cancer
- PMID: 32336671
- PMCID: PMC9375652
- DOI: 10.1016/j.jasc.2020.04.003
EBUS-FNA cytologic-histologic correlation of PD-L1 immunohistochemistry in non-small cell lung cancer
Abstract
Introduction: Immune checkpoint pathway markers induce immune tolerance to non-small cell lung cancer (NSCLC). Therapeutic antibodies targeting the programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway have demonstrated efficacy in tumors expressing relatively high PD-L1 levels. Minimally invasive endobronchial ultrasound-guided fine needle aspiration allows patients with inoperable tumors or comorbidities to attain a confirmatory diagnosis. The aims of the present study were to determine whether PD-L1 testing is equivalent to cytology and biopsy or resection specimens at different tumor proportion score cutoffs and for different NSCLC subtypes.
Materials and methods: Data were retrospectively collected for patients with paired NSCLC cytology and surgical resection specimens from May 4, 2007 to May 4, 2017. The Food and Drug Administration-approved Dako PD-L1 immunohistochemistry 22C3 pharmDx kit was used to measure PD-L1 on paired cytology cell block and biopsy or resection specimens, and the PD-L1 tumor proportion scores were recorded. Statistical analysis of categorical and continuous variables was performed using SAS, version 9.4.
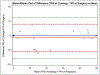
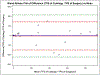
Results: A total of 53 paired cytology and resection samples (27 adenocarcinoma, 25 squamous cell carcinoma, and 1 unclassified) were analyzed. Supposing the resection specimen to reflect the true PD-L1 expression, the sensitivity, specificity, positive predictive value, negative predictive value, and overall agreement for the cytology method was 73.3%, 65.2%, 73.3%, 65.2%, and 69.8%, respectively. For high PD-L1 expression (≥50%), the cytology method demonstrated an overall agreement of 79.2%. The overall agreement between methods was 81.5% and 76% for cases of adenocarcinoma and squamous cell carcinoma, respectively.
Conclusions: NSCLC cytology samples from endobronchial ultrasound-guided fine needle aspiration are suitable for PD-L1 testing, especially using a high PD-L1 expression cutoff of ≥50% and for adenocarcinoma.
Keywords: Checkpoint inhibitor; EBUS-FNA; FNA; Non–small cell lung cancer; PD-L1.
Copyright © 2020 American Society of Cytopathology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Ilie M, Hofman V, Dietel M, Soria JC, Hofman P. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016;468(5):511–525. - PubMed
-
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

